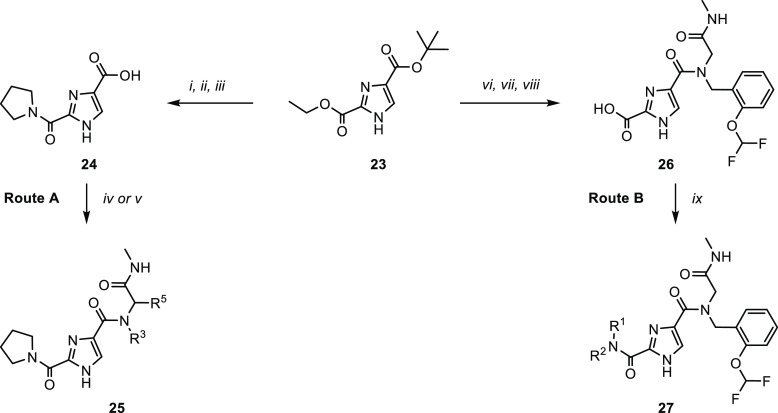
Scheme 1. Synthetic Routes (A, B) for the Synthesis of 2,4-1H-Imidazole Carboxamides.
Reagents and conditions: [i] LiOH, THF/H2O, RT, 16 h; [ii] pyrrolidine, HATU, DMF, RT, 16 h, 36% over two steps; [iii] formic acid, CHCl3, 2 h, reflux, 95%; [iv] amine, HATU, Et3N, DMF, RT, 16 h, 20–67%; [v] R3NH2, R5CHO, methylisocyanide, MeOH, RT, 16 h, 9–64%; [vi] formic acid, CHCl3, 2 h, reflux, 88%; [vii] 2-((2-(difluoromethoxy)benzyl)amino)-N-methylacetamide, HATU, Et3N, DMF, RT, 16 h, 77%; [viii] LiOH, THF/H2O, 40 °C, 16 h, 98%; [ix] R1R2NH, HATU, Et3N, DMF, RT, 16 h, 18–80%.