Table 1. In Vitro Biochemical Potency Data for Initial Derivatives of Compound 7.
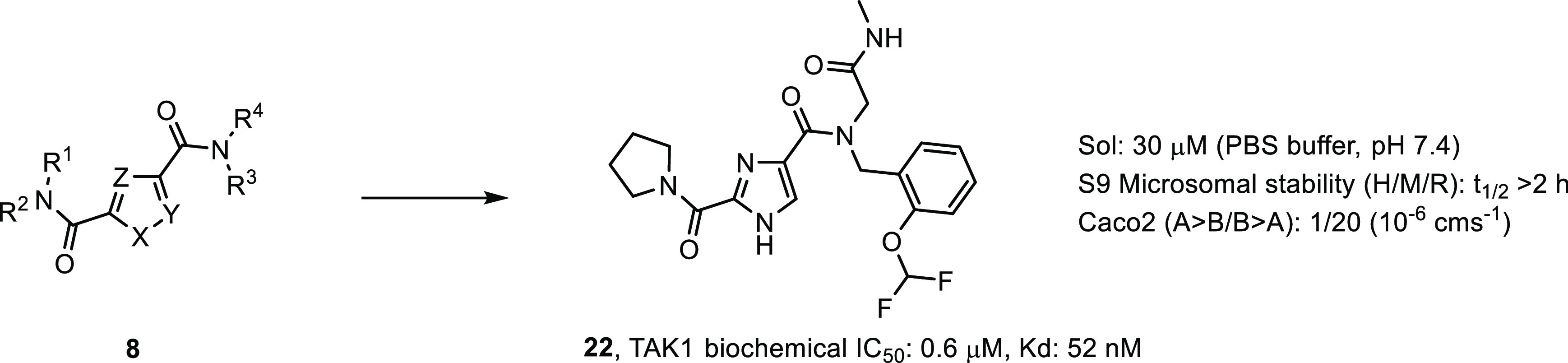
| Compd | R1, R2N | R3 | R4 | X, Y, Z | TAK1 IC50a |
|---|---|---|---|---|---|
| 9 | 1-pyrrolidinyl | 2-(OCHF2)benzyl | 2-amino-2-oxoethyl | N, CH, CH | 0.9 |
| 10 | 1-pyrrolidinyl | 2-(OCHF2)benzyl | 2-(isopropylamino)-2-oxoethyl | N, CH, CH | 1.7 |
| 11 | 1-pyrrolidinyl | 2-(OCHF2)benzyl | oxazol-2-ylmethyl | N, CH, CH | 3.2 |
| 12 | 1-pyrrolidinyl | 2-(OCHF2)benzyl | methyl | N, CH, CH | 3.5 |
| 13 | 1-pyrrolidinyl | 2-(OMe)benzyl | 2-(methylamino)-2-oxoethyl | N, CH, CH | 2.8 |
| 14 | 1-pyrrolidinyl | 2-(OEt)benzyl | 2-(methylamino)-2-oxoethyl | N, CH, CH | 2.6 |
| 15 | 1-pyrrolidinyl | (3,4-dihydro-2H-benzo[b][1,4]dioxepin-6-yl)methyl | 2-(methylamino)-2-oxoethyl | N, CH, CH | 3.4 |
| 16 | 1-pyrrolidinyl | (1H-indol-4-yl)methyl | 2-(methylamino)-2-oxoethyl | N, CH, CH | 1.8 |
| 17 | N,N-dimethyl | 2-(OCHF2)benzyl | 2-(methylamino)-2-oxoethyl | N, CH, CH | 26 |
| 18 | 1-piperidinyl | 2-(OCHF2)benzyl | 2-(methylamino)-2-oxoethyl | N, CH, CH | 1.8 |
| 19 | N-ethyl | 2-(OCHF2)benzyl | 2-(methylamino)-2-oxoethyl | N, CH, CH | >100 |
| 20 | 1-pyrrolidinyl | 2-(OCHF2)benzyl | 2-(methylamino)-2-oxoethyl | N, N, CH | 59 |
| 21 | 1-pyrrolidinyl | 2-(OCHF2)benzyl | 2-(methylamino)-2-oxoethyl | NMe, CH, CH | >100 |
Biochemical Lanthascreen assay with TAK1–TAB1 fusion protein in the presence of 10 μM ATP, mean IC50 values in μM, n = 2.
