Abstract
Photoacoustic imaging (PAI), featuring rich contrast, high spatial/temporal resolution and deep penetration, is one of the fastest-growing biomedical imaging technology over the last decade. To date, numbers of handheld and semi-handheld photoacoustic imaging devices have been reported with corresponding potential clinical applications. Here, we summarize emerged handheld and semi-handheld systems in terms of photoacoustic computed tomography (PACT), optoacoustic mesoscopy (OAMes), and photoacoustic microscopy (PAM). We will discuss each modality in three aspects: laser delivery, scanning protocol, and acoustic detection. Besides new technical developments, we also review the associated clinical studies, and the advantages/disadvantages of these new techniques. In the end, we propose the challenges and perspectives of miniaturized PAI in the future.
Keywords: Photoacoustic computed tomography, Optoacoustic mesoscopy, Photoacoustic microscopy, Handheld and semi-handheld photoacoustic imaging
1. Introduction
Photoacoustic imaging (PAI) has emerged as one of the fastest growing biomedical imaging techniques in the last decade [1,2]. Briefly, as the delivery of a pulsed/modulated laser beam to the biological tissue, exogenous or endogenous chromophores will absorb the photons and convert the photon energy into heat. This conversion will induce rapid temperature change, generate thermoelastic expansion, and then radiate the acoustic wave out of the biological tissue, referred to as photoacoustic (PA) signal [3]. The principle of PAI shows that it is a hybrid imaging modality combining the advantages of both optics and ultrasound, such as rich endogenous contrast, high spatial resolution, and deep tissue penetration [[1], [2], [3], [4], [5]].
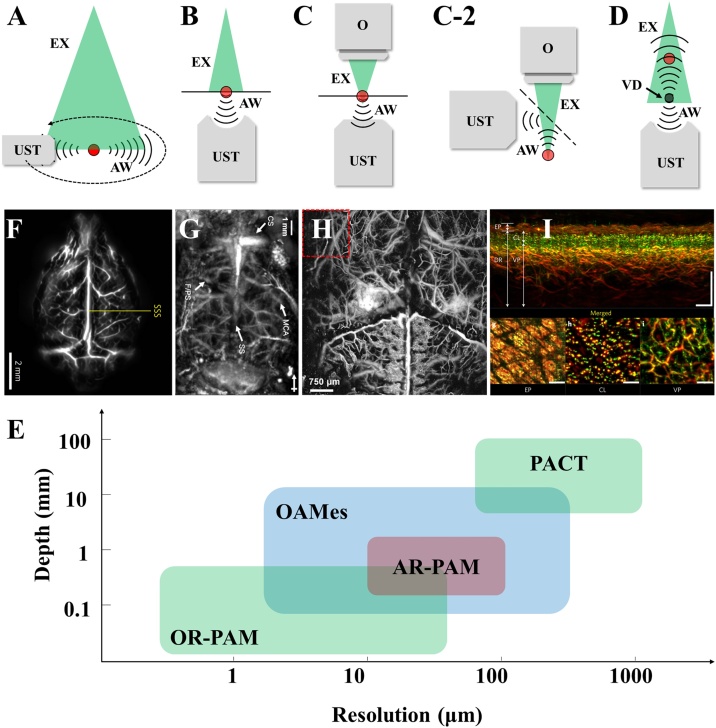
In terms of system configurations, PAI consists of two main sub-modalities: photoacoustic computed tomography (PACT) and photoacoustic microscopy (PAM) presented by Fig. 1(A) to (H) [[3], [4], [5]]. As shown in Fig. 1(A), PACT employs a full-field and uniformly distributed laser illumination to induce photoacoustic effect over the entire region of interest. The transducer with a high sensitivity and large directivity is preferred in PACT to receive PA signals over the entire imaging domain with a higher signal-to-noise-ratio (SNR) and less artifacts [6,7]. A full-view (360°) recording of PA signals by either scanning a single detector or employing a transducer array forms the map of PA sources using mathematical reconstruction methods, such as delay-and-sum, filtered back projection, time-reversal algorithm, and model-based reconstruction methods [[8], [9], [10], [11], [12]]. Comparing with PACT, PAM can provide a higher spatial resolution by employing higher frequency ultrasound transducer or focusing the excited laser beam, referred to as acoustic resolution photoacoustic microscopy (AR-PAM) and optical resolution photoacoustic microscopy (OR-PAM), respectively [[13], [14], [15]]. AR-PAMs use full-field illumination or weakly focused light to improve the energy density. A focused ultrasonic transducer (UST) is usually used to detect PA signals, and the acoustic focus of UST is smaller than the area illuminated by the pulsed laser beam as shown in Fig. 1(B) (Transmitted illumination, the light illumination and ultrasonic detection are distributed on both sides of the target). Hence the lateral resolution of AR-PAM is determined by the acoustic focus. On the contrary, in OR-PAM (Fig. 1(C)), the excited laser focus is smaller than the acoustic focus by using an optical objective/scan lens (Fig. 1(C)). Hence, comparing with AR-PAM, OR-PAM is able to achieve a higher lateral resolution by sacrificing the penetration depth. Since the transmitted illumination is difficult to image thick samples, epi-illumination OR-PAM (Fig. 1(C-2), the light illumination and ultrasound detection are distributed on the same side of the target) is more convenient in biomedical and clinical studies. Apart from AR-PAM and OR-PAM, optoacoustic mesoscopy (OAMes) has been described as a bridge between PACT and PAM by introducing the concept of ‘virtual detector’ [16,17]. In OAMes, the focal point of the ultrasound detector is assumed to be a virtual detector with a small effective collection area but a higher sensitivity compared with the conventional UST (Fig. 1(D)). Although there are no clear boundaries between PACT, PAM, and OAMes, we can still categorize them with specific imaging features as shown in Fig. 1(I). PACT employs high energy lasers with a pulse energy of tens of millijoules to cover the entire field of view (FOV). The pulsed width of PACT laser is usually tens to hundreds of nanoseconds and the pulse repetition rate is usually 10–100 Hz [1,2]. The wide pulsed duration causes a low-frequency PA excitation. By using the UST or UST arrays in the MHz scale, PACT achieves spatial resolutions of hundreds of microns and a centimeter-scale penetration, enabling non-invasive imaging of deep tissues as shown in Fig. 1(E) [3]. PAM prefers a pulsed laser with a high repetition rate of a few hundred kilohertz, and the pulsed duration is below 20 ns in general. The UST with a high center frequency and wide bandwidth from tens to hundreds of megahertz is required. Optical/acoustic focus induces a high spatial resolution around but a superficial imaging depth compared with PACT. By using advanced techniques such as objective lenses with high numerical apertures, optical detection with a ultrawide bandwidth, and super-resolution imaging strategies, PAMs are able to capture single capillary and visualize microvasculature both in animals and human beings as shown in Fig. 1(F) and (G) [18,19]. OAMes bridges the gap between PACT and PAM by employing UST with a bandwidth from 10 to 200 MHz, thus leading to a compromising penetration (0−10 mm) and spatial resolution (10−100 μm) between PACT and PAM. Similar to PACT, a reconstruction algorithm is required in OAMes since the transducer captures the PA signals from the entire illumination area. Similar to PACT, the frame rate of OAMes can be improved with transducer array. However, it is challenging to manufacture transducer arrays with a center frequency and bandwidth over 100 MHz. Besides, the spherical focused geometry is not applicable to the detection elements and the limited active detection area is also against the optimization of OAMes performance [49]. Hence, OAMes, especially raster scanning OAMes (RSOM) prefers a single UST instead of transducer arrays with a high center frequency, ultra-wide bandwidth, and large active detection area. Due to the noninvasiveness, high resolution, and label-free imaging of blood vessels within a moderate penetration depth, OAMes is considered as a potential approach especially in quantifying skin morphology and inflammatory landmarks as shown in Fig. 1(H) [20].
Fig. 1.
Representative implementations of PAT. (A) PACT. (B) AR-PAM. (C) and (C-2) Transmitted and epi-illumination OR-PAM. (D) OAMes. EX, excitation light. AW, acoustic wave. UST, ultrasonic transducer. VD, virtual detector. (E) Cerebral vasculature of a rat obtained by PACT (11 mm in depth). [3]. (F) In vivo AR-PAM imaging of a mouse brain (3.2 mm in depth) [18]. (G) Cerebral vasculature of a mouse obtained by high resolution OR-PAM (more than 200 μm in depth) [19]. (H) The cross-section image (top) of healthy skin and MAPs of different skin layers (bottom). EP, epidermis. CL, capillary loops. VP, vascular plexus. DR, dermis (5 mm in depth) [19]. (I) Comparison of resolution and penetration depth between PACT, OAMes, and PAM. Reprinted with permission from Ref. [3,[18], [19], [20]].
Over the last two decades, PAI has been used in numerous studies such as whole body imaging of small animals [3,21,22], in vivo vascular imaging in various organs of rodents and primates [23,24], and studying neurovascular coupling [25,26]. In addition, potential clinical applications include angiogenesis and vascular distribution inside/around tumors [27,28], retinal vasculature associated with diabetic retinopathy [29,30], intestinal diseases such as Crohn’s disease and mesenteric venous thrombosis [31,32], vascular lipid plaque [33,34], and breast cancer [27,[35], [36], [37]] as shown in Table 1. Although PAI has multi-scale and multi-parametric imaging capability with various advanced features, early-stage PAI systems suffer from several limitations. For example, it is hard to simultaneously satisfy the portable design of system and high image performance such as large FOV, fast image speed, and high spatial resolution at micron level, making it inaccessible to many clinical or fundamental applications with the requirement of adaptability. In most PAI systems, optical parametric oscillator, dye-based lasers, or Nd:YAG lasers are necessary to induced PA signals, however, all of these are bulky and expensive [35,38,39]. To overcome these limitations and expand the applicable scenarios of PAI, handheld or semi-handheld platform is a promising solution. In general, handheld/semi-handheld PAI systems are defined as imaging platforms containing an imaging head without supporting structure or connected to an articulated arm, which can be manipulated by the operator [40]. Considering the detailed configuration, all the PAIs includes three primary parts: laser delivery, scanning protocol and acoustic detection. To miniaturize the imaging head and realize handheld design, it is required to propose new strategies in either one or more of these three primary parts. For example, in PACT (Table 1, part 1), it is feasible to miniaturize the imaging interface by employing optical fiber bundles and ultrasound transducer arrays [[41], [42], [43]]. In PAM (Table 1, part 3), miniaturization requires the use of small ultrasound transducers and new scanning protocols such as rotatory scanning mechanisms, two-dimensional (2D) galvanometer scanners and micro-electro-mechanical system (MEMS) mirrors [[44], [45], [46]]. For OAMes (Table 1, part 2), raster scanning mechanism is employed instead of scanning along circular orbit to realize handheld/ semi-handheld configuration [20,47]. With the recent explosion of handheld/ semi-handheld PAI studies [40,41,48,49], this review aims to summarize recent developments and clinical applications, especially recently reported handheld/semi-handheld OR-PAM, providing insight into the future progress of PAI.
Table 1.
Specifications of typical handheld and semi-handheld PAI systems. SLN, sentinel lymph nodes. NM, did not mention in the article. MMF, multimode fiber. SMF, single mode fiber. UST, ultrasonic transducer. L, lateral resolution. A, axial resolution. FOV, field of view.
| Clinical applications | Volume | Laser delivery |
Scanning protocol |
Acoustic detection |
Imaging performance |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pulse Repetition Frequency | Wavelength | Pulse energy | Delivery device | Scanning mechanism | Scanning device | UST | Center frequency | Bandwidth | Resolution | Penetration depth | FOV | Imaging speed/time | |||||||||||
| Part 1 PACT | [65] | Imaging guided SLN biopsy, human SLN imaging | 5.4 ×4 ×10 mm3 | 20 Hz | 780 nm | 17 mJ (probe output) | MMF | Without scanning | Linear array with 128 elements | 7 MHz | NM | L: 532.5 μm | > 6 cm | NM (B-scan mode) | 20 Hz | ||||||||
| A: 285 μm | |||||||||||||||||||||||
| [67] | Imaging guided biopsy, imaging of human finger and wrist | NM | 1-4 kHz | 850 nm | 200 μJ/row × 4 rows | light-emitting diodes | Without scanning | Linear array with 128 elements | 9 MHz | 77% | L: 0.46 mm | 4 cm | NM (B-scan mode) | 1.5-30 Hz with averaging | |||||||||
| A: 0.22 mm | |||||||||||||||||||||||
| [71] | Dorsalis pedis artery, tibialis posterior artery, Crohn’s disease, thyroid lobes, muscle, breast | NM | 10-50 Hz | 730, 750, 800, 830 nm | According to laser safety standard (IEC 60825–1) | Fiber bundle | Without scanning | Curved array with 128 elements | 5/7.5/8 MHz | NM | 200 μm | > 2 cm | 40×40 mm2 (B-scan mode) | 5-10 Hz | |||||||||
| [78] | Breast imaging, thyroid lobes, human skin, human extremities | NM | Up to 100 Hz | 690-900 nm | Below 20 mJ/cm2, (sample surface) | Fiber bundle | Without scanning | Spherical array with 256 elements | 4 MHz | 100% | 200 μm | ∼ 2cm | 14×14 ×7 mm3 | 10 Hz | |||||||||
| [81] | Imaging guided SLN biopsy, monitoring of photothermal therapy | 80 ×25 ×24 mm3 21 g | CW | 450, 638, 808 nm | 3.5, 2.2, and 2.0 W, respectively | Laser diodes | Without scanning | Planar array with 72 elements | 2.25 MHz | 60% | 0.74 mm | > 5 mm | 10×10 ×10 mm3 | 3 Hz | |||||||||
| [82] | Human arm vasculature, imaging guided SLN biopsy | 44 g | 10 Hz | NM | 14.7 mJ (fiber output) | MMF | Without scanning | Planar array with 72 elements | 2.25 MHz | 60% | 0.73-0.8 mm | > 1 cm | 10×10 ×10 mm3 | 10 Hz | |||||||||
| [92] | Dorsalis pedis artery, fingertip, human skin | NM | 30 Hz | 750 nm | 6.5 mJ (sample surface) | Fiber bundle | Raster scanning | Galvanometer | FP chamber | NM | 30 MHz | 75-125 μm | ∼ 14 mm | 1-2 cm in x, y | 90 s | ||||||||
| Part 2 OAMes | [20] | Human skin | NM | 500 Hz, up to 2 kHz | 532 nm/ NIR | 1 mJ (laser output) | Fiber bundle | Raster scanning | Two motorized stages | Spherically focused UST | 100 MHz | 10-180 MHz | L: 18 μm | ∼ 4 mm | 4×2 mm2 in lateral | 70 s | |||||||
| A: 4 μm | |||||||||||||||||||||||
| [47] | Nailfold microvasculature | NM | 500 Hz | 532 nm | 1 mJ (laser output) | Fiber bundle | Raster scanning | Two motorized stages | Spherically focused UST | 50 MHz | 10-120 MHz | L: 30 μm | ∼ 5 mm | 4×2 mm2 in lateral | 70 s | ||||||||
| A: 8 μm | |||||||||||||||||||||||
| Part 3 PAM | [101] | Melanoma | NM | 10 Hz | 650 nm | 15 mJ/cm2 (sample surface) | Fiber bundle | Raster scanning | Motorized stage | Spherically focused UST | 25 MHz | 100% | L: 230 μm | ∼ 4mm | 10 mm in length (B-scam mode) | 10 s (B-scan) | |||||||
| A: 59 μm | |||||||||||||||||||||||
| [102] | Oral imaging, human skin | NM | 10 kHz | 532 nm | 80 nJ (sample surface) | SMF | Raster scanning | Galvanometer | Flat responsive UST | 15 MHz | NM | L: 8.9 μm | ∼ 2.4 mm | 2×2 mm2 in lateral | 16 s | ||||||||
| A: 113 μm | |||||||||||||||||||||||
| [103] | Oral imaging, cancer monitoring | 170 × 135 × 140 mm3 | Up to 600kHz | 532 nm | 80 nJ (sample surface) | SMF | Rotatory scanning | Galvanometer | Cylindrically focused UST | 15/25 MHz | 75% | L: 4.2/10.8 μm | ∼ 800 μm | φ 8.4mm in lateral and 1.5 mm in axial | 5 s | ||||||||
| A: 60/90 μm | |||||||||||||||||||||||
| [106] | Oral imaging, multi-organs imaging of animals | 22 × 30 × 15 mm3 20 g | 50 kHz | 532 nm | 80 nJ (sample surface) | SMF | Raster scanning | Electrothermal MEMS | Flat responsive UST | 10 MHz | 80% | L: 3.8 μm | NM | 2×2 mm2 in lateral | 3.2 Hz | ||||||||
| A: 105 μm | |||||||||||||||||||||||
| [113] | Oral imaging | Diameter: 2.4 mm | 50 kHz | 532 nm | 80 nJ (sample surface) | SMF | Raster scanning | Electrostatic MEMS | cylindrical planar UST | 10 MHz | NM | L: 18.2 μm | ∼ 800 μm | φ 2.4mm in lateral | 4 Hz | ||||||||
| A: 137.4 μm | |||||||||||||||||||||||
| [114] | Human skin, mole | 80 × 115 × 150 mm3 | 88 kHz | 532 nm | 18 mJ/cm2 (sample surface) | SMF | Raster scanning | Electromagnetic MEMS | Flat responsive UST with acoustic lens | 50 MHz | NM | L: 5.0 μm | ∼ 540 μm | 2.5×5 ×0.5 mm3 | 2 Hz | ||||||||
| A: 26 μm | |||||||||||||||||||||||
| [115] | Melanoma, oral imaging | Diameter: 17mm, length: 31 mm; 162 g | 50 kHz | 532 nm | 8.6 mJ/cm2 (sample surface) | SMF | Raster scanning | Electromagnetic MEMS | Flat responsive UST with acoustic lens | 50 MHz | NM | L: 12 μm | ∼ 800 μm | 2×2 mm2 in lateral (typical) | 0.05 Hz | ||||||||
| A: 30 μm | |||||||||||||||||||||||
2. Progress in handheld and semi-handheld PACT
PACT owns a centimeter-scale penetration depth, a regular spatial resolution from tens of to hundreds of microns, and functional/metabolic imaging capability (average ∼3 cm, Table 1, part 1) [3]. By injection of exogenous chromophore and localization, super-resolution PACT can even achieve nanoscale spatial resolution [50]. The conventional setups for PACT usually employ a single-element transducer to capture the time-resolved PA signals and a motorized rotator to scan the transducer in a full-view (360°) circular orbit. [51]. In conventional PACTs, it is preferred to capture PA signals with more detection angles and carry out averaging to eliminate the background noise, leading to time consuming and bulky size. Hence, it is difficult to achieve compact design and high temporal resolution, which are quite essential in both clinical and fundamental applications (For example, a system with a pulsed repetition rate of 100 Hz and a scanning step of 1°will cost 3.6 s for one image, while the array-based system can capture with a frame rate of 100 Hz). To accelerate imaging speed of PACT, full-ring transducer arrays are always introduced to replace circular scanning as shown in Fig. 1(A) [3,35,52,53]. By using transducer arrays, PACT can capture PA signals in different views for each single laser pulse with a cost of detection bandwidth (single element transducer: up to hundreds of MHz; transducer array: tens of MHz), leading to a higher temporal resolution (10−200 Hz, or frames per second) and a lower spatial resolution. Full-ring-array-based PACT can receive PA signals over a view of 360° to eliminate artifacts and improve SNR, which has been extensively applied in detecting breast cancerous lesions [35,53], evaluating human joint diseases [52,54,55], and visualizing vasculatures in human extremities [56]. However, due to the configuration of full-ring transducer arrays, it is hard to design handheld or semi-handheld devices. In contrast, transducer arrays with linear (Fig. 2(A)), curved (Fig. 2(B)), spherical (Fig. 2(C)), and planar (Fig. 2(D)) shapes are widely used in handheld platforms in conjunction with laser delivery based on optical fibers or fiber bundles. In this section, we focus on various handheld PACT systems utilizing linear, curved, and spherical transducer arrays as well as the associated potential clinical applications.
Fig. 2.
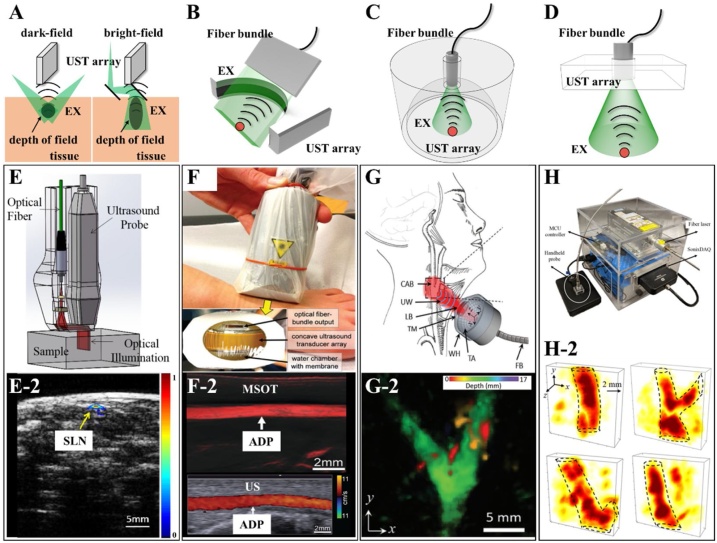
Typical handheld PACTs based on UST arrays with various shapes and corresponding imaging results. (A—D) Depiction of PACT systems that use different UST arrays. (A) Linear array. (B) Curved array. (C) Spherical array. (D) Two-dimensional planar array. (E) Schematic of a handheld PACT probe based on a commercial ultrasound system with a linear UST array [65]. (E-2) Emerged PA&US image (right) of the sentinel lymph nodes (SLN) in a rat [65]. (F) Photographs of a handheld probe used in the multi-spectral optoacoustic tomography (MSOT) system. The inset image is the front view of the probe consisting of a concave UST array with 128 elements, an optical fiber bundle, and a water chamber sealed by plastic membrane to achieve ultrasonic coupling [71]. (F-2) MSOT image (top) with an excitation wavelength of 830 nm and duplex US image (bottom). ADP, dorsalis pedis artery [71] (G) A diagram of in vivo imaging of carotid artery with a handheld PACT probe that consists of a laser delivery fiber bundle, a two-dimensional spherical ultrasound transducer. CAB, carotid artery bifurcation. FB, fiber bundle. LB, laser beam. TA, transducer array. TM, transparent membrane. UW, ultrasound waves. WH, water holder [78]. (G-2) Volumetric imaging of carotid arteries of a volunteer with the proposed handheld PACT in (G) [78]. (H) A photograph of a handheld probe and whole portable system with a size of 25 25 20 cm3. The handheld probe consists of a 2D UST array with 72 elements and an optical window in the center, an optical diffuser and a laser delivery system [82]. (H-2) Three-dimensional (3D) imaging results of blood vessels in human arm [82]. Reprinted with permission from Ref. [65,71,78,82].
2.1. Linear transducer array
Medical ultrasonography (US), one of the most important clinical imaging modalities, usually uses an ultrasound transducer array to generate ultrasound pulses and receive echoes in real-time. There are many types of transducer arrays used for different scenarios, such as linear arrays for imaging superficial small organs, convex arrays for abdominal imaging, and cavity arrays for endoscopic inspection. Based on the principle of PAI, it is easy to integrate PACT into clinical US platform by providing additional laser delivery systems. This integration makes PACT compact and portable for clinical applications. Fig. 2(A) presents a typical PACT system based on a linear array of medical US, in which optical fibers/fiber bundles are usually employed to deliver laser energy to the sample. Various handheld PACTs have been proposed based on this configuration [[57], [58], [59], [60], [61], [62], [63]]. Most of reported PA-US systems used dark-field illumination configurations since the piezoelectric transducer is opaque (Fig. 2(A) left). It is a convenient way to deliver the oblique incidence laser pulses to the imaging plane using optical fibers or fiber bundles from the side of the transducer array. By using dark-field illumination, PAI can avoid the strong signals from the surface and enhance the SNR of deep vessels. However, the laser energy is confined in a specific depth within the sample. Thus, bright-field illumination is proposed to extend the depth of field (Fig. 2(A) right). Wang et al. developed a handheld PACT system based on a medical US system (CL15-7, Philips) with a linear array [64]. The array and optical delivery fiber bundles are assembled with a right angle, an optical transparent acoustic reflector was mounted at an angle of 45°in the center to merge the light and ultrasound beam. Song et al. proposed a handheld dual-modality PA/US system using the strategy shown in Fig. 2(E) [65]. A linear array with 128 elements and a center frequency of 7 MHz was employed for ultrasonic detection. An optical system consisting of a multi-mode fiber, two cylindrical lenses, and a polymethyl methacrylate optical/acoustic coupler was used to deliver the laser pulses with a pulse repetition rate of 20 Hz to the samples. With the specific illumination strategy, the handheld probe can resolve blood vessels located at a varied depth from 0.1 mm to ∼20 mm. In vivo imaging of Indocyanine green (ICG) labeled sentinel lymph nodes of rats demonstrated the clinical potential of this probe as shown in Fig. 2(E-2). By selecting the optimal excitation wavelength of ICG, PACT clearly showed the positions and morphologies of the target. With additional structural information provided by US imaging, the handheld system was applied to guide the biopsies of sentinel lymph nodes using co-registered real-time PA/US images. In addition to optical fibers/fiber bundles, an alternative way to excite PA signals in handheld design is to use laser diodes (LD) or laser-emitting diodes (LED) [66,67]. Compared with Optical Parametric Oscillator, dye-based lasers, or Nd:YAG lasers, LD and LED are much smaller and lighter with a higher pulse repetition rate. Steenbergen et al. developed a handheld PA/US system by integrating medical US and LDs with a pulse energy of 0.56 mJ and a repetition rate of 10 kHz [66]. Xia et al. proposed a study of human fingers with a commercial LED-based PA/US system (AcousticX, PreXion) [67]. The system employed a 128-element linear array and four LED arrays with a pulse energy of 200 μJ and a high repetition rate of up to 4 kHz. Although the progress of LD/LED-based PACT is exciting, it is still challenging to increase the output energy, shorten the pulse duration and improve the beam quality of LD/LED without scarifying the portability.
2.2. Curved transducer array
Although the linear-array-based PACT can improve the temporal resolution by avoiding mechanical scanning of transducer in conventional PACTs, it has several inherent limitations such as radial artifacts and morphological distortions due to the limited detecting views [41]. Compared to the linear array, the cylindrically focused curved array is able to cover more detecting views and eliminate artifacts/distortions, which make curved array more suitable for clinical application such as imaging of deep subcutaneous tissues [31,[68], [69], [70], [71]]. Ntziachristos et al. developed the first handheld multi-spectral optoacoustic tomography (MSOT) system with a cylindrically focused curved array [68]. The ultrasound array has a center frequency of 8 MHz, a total number of 128 elements with a focal length of 2 cm, and a fan-shaped view of 135°. Similar to previous PA/US systems and handheld MOST, the laser pulses were delivered by the optical fiber bundles positioned at both sides of the transducer array. The MSOT systems realizes ultrafast PA-US imaging by utilizing a laser with a pulsed repetition rate of 50 Hz and achieves multispectral functional imaging via a fast-tunable pulsed laser. In addition, they proposed various special handheld MOST systems and mathematical algorithms for specific clinical and fundamental applications, such as muscle oxygenation monitoring [69], in vivo imaging of human thyroid [70], imaging of blood vessels in human foot [71], and assessment of Crohn’s disease [31]. Fig. 2(F) shows a procedure of human dorsalis pedis artery imaging with a handheld MOST reported in 2016 [71]. Fig. 2(F-2) presents the MSOT PA image and contrastive duplex US images of the vasculature on a foot, which shows the dorsalis pedis artery. Furthermore, to combine the advantages of linear array with extended FOV and curved array with a better imaging performance, Razansky et al. proposed a hybrid-array-based optoacoustic and ultrasound system [72]. The array consists of a linear array with 128 elements in the center and two concave segments with total 128 elements in the sides of linear array, resulting a large FOV of 40 × 40 mm2 and a high resolution of ∼110 μm.
2.3. Spherical transducer array
By using the one-dimensional (1D) transducer array, the portable PACT is only able to recover cross-sectional images in real-time, referred to as B-scan. Additional scanning is required to form a 3D image. Compared with 1D arrays, spherical arrays enable real-time volumetric imaging [73]. Razansky et al. firstly implemented a spherical array with 256 elements in a handheld PACT. The elements with a center frequency of 4 MHz, a bandwidth of 100 %, were disposed on a spherical surface with a radius of 40 mm, covering an angle of 90° [74,75]. The laser pulses were delivered via a fiber bundle through the hole in the center of the array. A transparent polyethylene membrane was used to protect the transducer surface, and the gap was filled with the coupling medium for acoustic propagation. A series of clinical and fundamental studies have been proposed including human skin imaging [75], visualization of vasculature in human extremities [76], breast cancer detection [77], and evaluation of thyroid lobes [78]. Fig. 2(G) shows a diagram of human thyroid lobes imaged with a handheld PACT based on the spherical array (2019) [78]. A pulsed laser with a tunable wavelength range of 680−950 nm and a high pulsed repetition rate of 100 Hz was employed in this system. To ensure the laser safety, the repetition rate was finally set to 10 Hz. Fig. 2(G-2) shows a maximum amplitude projection (MAP) image of the carotid artery bifurcation of a volunteer. Although the spherical array covers more views compared with linear/curved array, in addition to the limited FOV, it is difficult to form a homogeneous illumination pattern on the sample surface. Moreover, spherical array is not a standard probe in medical US and the applicable scenario is limited [73]. One of the potential scenarios might be volumetric visualization of superficial vascular and chromophores in soft tissue, such as dermatology, subcutaneous fat and lymphatic tissue, and breast.
2.4. 2D planar array
Besides the limited FOV and the challenge for light delivery, a membrane-sealed chamber filled with coupling medium is necessary in spherical-array-based handheld PACT as shown in Fig. 2(F). A practical solution is 2D planar array as shown in Fig. 2(D) [[79], [80], [81], [82]]. Wang et al. proposed a handheld PACT based on a clinical planar array (iU22, Philips Healthcare), demonstrated the clinical potential using in vivo imaging guided the biopsy of the sentinel lymph nodes in mice [79]. Similar to linear-array-base PACT, LDs and LEDs have been implemented in planar-array-based PACT to achieve compact design and high-speed imaging. Zheng et al. proposed a compact PACT integrating LDs and a planar array [81]. Three compact continuous-wave LDs with wavelengths of 450, 638, and 808 nm were used instead of conventional optical fiber to achieve multi-spectral coherent frequency-domain PAI. The weight was significantly reduced to 21 g compared with conventional handheld probes, which can be applied in wearable healthcare. However, the imaging performance was unsatisfying due to the relative low laser energy. Therefore, Zheng. et al. developed an optimized PACT system containing a handheld imaging head weighting 44 g and equipped with a 15-mJ fiber-coupled laser system (CNI LASER, China) as shown in Fig. 2(H) [82]. To efficiently deliver and diffuse the laser energy to the sample surface, a fiber-coupled collimator, a micro condenser lens, and an optical diffuser were employed in the probe. The customized diffuser serves as the acoustic delay line for acoustic detection. A two-dimensional planar array with 72 piezoelectric elements, an optical window, a center frequency of 2.25 MHz, and a bandwidth of 65 % was employed to detect PA signal. Fig. 2(H-2) shows the 3D rendering of human blood vessels.
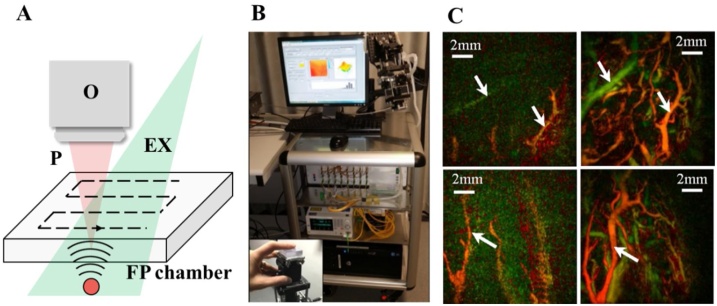
Apart from conventional PAI based on piezoelectric transducers, some novel optical methods have been proposed to detect PA signals such as Fabry–Pérot [83,84], akinetic probe [85], and micro-ring resonators [86,87]. Fig. 3(A) shows the principles of acoustic detection in all-optical PACT system based on Fabry–Pérot chamber [83]. The excitation light passed through the Fabry–Pérot detector to achieve full-field illumination of the chromophores inside the sample without any attenuation due to the transparency of the detector to the excitation wavelength. PA signals induced by the excitation light propagate to the tissue-detector interface, result in the change of the Fabry–Pérot cavity due to the vibration, and thus lead to the variation of interference intensity of the probe laser beam. By scanning the probe beam two-dimensionally in the surface of the chamber, a volumetric PA image can be reconstructed. Although the probe beam is scanned in a form of point-by point, which is similar to the scanning mechanism of PAM, the detected PA signal in each point is contributed by all the chromophores in the imaging domain. Hence, the Fabry–Pérot acts as a two-dimensional planar array in this configuration and a reconstruction algorithm similar to conventional PACTs is required. By using optical detection, all-optical PAI has a better spatial resolution but sacrifices penetration depth because of the tight aperture and wide detection bandwidth (Table 1, part 1, lines 7) compared with PACT based on piezoelectric devices (Table 1, part 1, lines 1–6). It is noted that Beard et al. have proposed a series of PACT prototypes based on Fabry–Pérot sensors [[88], [89], [90], [91], [92]]. Fig. 3(B) presents a semi-handheld all-optical PACT system, which is mounted on an articulated arm to provide multi-degree freedom for accessing the dorsalis pedis artery and fingertip in different states, i.e. stimulation in terms of immersion in cold (left hand panels) and warm (right hand panels) water (Fig. 3(C)). Using the articulated probe, the system can facilitate the imaging examination of different regions of interest over the entire human body to assist the clinical diagnosis [92]. Since the Fabry–Pérot sensor is transparent, the developed all-optical PACT systems are easily combined with optical coherence tomography (OCT) to provide both functional and morphological information of the biological tissues [90,91].
Fig. 3.
All-optical PACT with a 2D planar Fabry–Pérot chamber and the corresponding results. (A) The schematic of all-optical PACT, EX, excitation light. O, objective lens. P, probe laser beam. FP chamber, Fabry–Pérot chamber. (B) A photograph of an all-optical PACT system with an articulated arm, the inset presents an imaging process of fingertip [92]. (C) MAP images of three fingertips after cold (left column) and hot (right column) stimulations. There are significant morphological changes in vascular after stimulations. The white arrows indicate blood vessels in different conditions [92]. Reprinted with permission from Ref. [92].
3. Progress in handheld and semi-handheld RSOM
OAMes aims to balance the penetration depth and resolution, focuses on potential applications with a depth ranging from 0 to 10 mm and a spatial resolution of tens of microns compared with PACT [49]. Fig. 4(A) illustrates the imaging strategy of raster scanning RSOM. Optical fibers or fiber bundles are usually used to deliver the laser pulses and form broad beam epi-illumination. The absorbers located close to and outside of the acoustic focus are recovered with a synthetic aperture focusing technique termed as ‘virtual detector’ [93,94]. In RSOM, the resolution is determined by the acoustic diffraction. Therefore, an ultra-wideband UST with a bandwidth of 100–200 MHz is generally employed to achieve a high spatial resolution. Compared with PACT (∼ 3 cm imaging depth and ∼200 μm resolution, Table 1, part 1) and PAM (∼1 mm imaging depth and ∼5 μm lateral resolution, Table 1, part 1), RSOM provides a compromise spatial resolution and penetration depth, which is suitable for high resolution imaging of vasculature in deep tissues (4 mm imaging depth and spatial resolution of ∼15 μm, Table 1, part 2) [16,95].
Fig. 4.
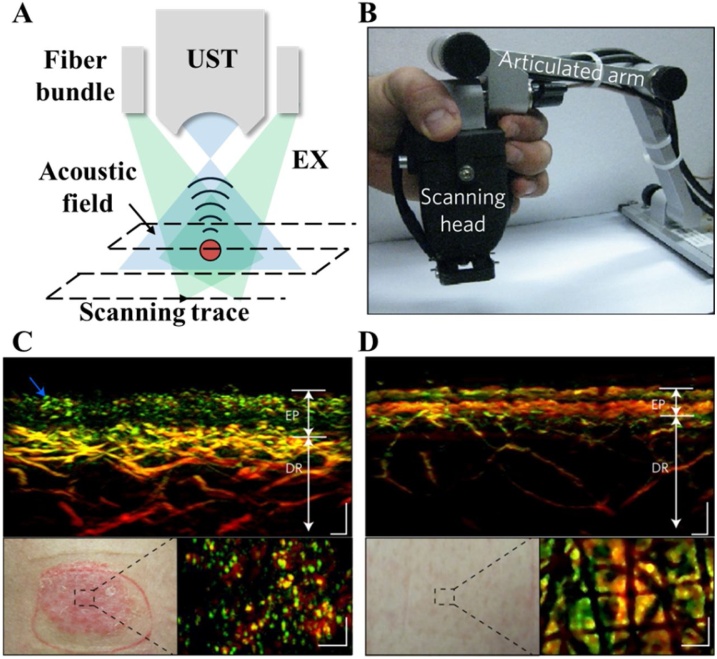
OAMes system and the corresponding results. (A) The schematic of OAMes, EX, excitation light. UST, ultrasonic transducer. (B) A photograph of a semi-handheld OAMes system consist with an articulated arm and s scanning head [20]. (C) The cross-section image of psoriatic skin, the blue arrow indicates the top part of elongated capillary loops (upper). Scale bars, 200 μm. A photograph of the psoriatic skin region (lower left) and the coronal slice of the epidermal region (lower right) Scale bars, 300 μm [20]. (D) The cross-section image of adjacent healthy skin (top). Scale bars, 200 μm. A photograph of the healthy skin region (lower left) and the coronal slice of the epidermal region (lower right) Scale bars, 300 μm [20]. EP, epidermis. DR, dermis. Reprinted with permission from Ref. [20]. (For interpretation of the references to colour in the Figure, the reader is referred to the web version of this article).
Ntziachristos et al. have proposed series of OAMes and RSOM studies in clinical applications, such as skin imaging of healthy volunteers [16], monitoring of vascular-targeted therapies [96], label-free visualization of psoriasis [20], and nailfold microvascular structure assessment [47]. Fig. 4(B) shows a semi-handheld RSOM system assisting with an articulated arm reported in 2017 [20]. A spherically focused transducer with a bandwidth of 10−180 MHz was employed. Due to the ultrawide detection bandwidth, the probe features high spatial resolutions of 18.4 in lateral and 4.5 μm in axial. Two motorized stages were employed in the semi-handheld head to carry out raster scanning as shown in Fig. 4(A). A customized fiber bundle with two distal ports was used to deliver the laser beam with a repetition rate of 2 kHz. Fig. 4(C) and (D) present a potential clinical application of the semi-handheld RSOM. Due to the capability of high resolution in dermis, RSOM can reveal the structural differences between psoriatic (Fig. 4(C)) and normal (Fig. 4(D)) skin. In 2018, a similar semi-handheld RSOM system was developed using a center frequency of 55 MHz. With a lower center frequency and ultrawide bandwidth from 10 to 120 MHz, the proposed RSOM provides a deeper penetration depth and a higher SNR without sacrificing the spatial resolution [96].
4. Progress in handheld and semi-handheld PAM
Featuring high resolution and rich contrast, PAM is able to provide structural, functional, and metabolic information of biological tissues [97,98]. Traditional PAMs usually use bulky motor stages to realize 2D scanning, making it difficult to achieve small, light, and fast imaging devices [99]. Various PAMs have been proposed by adopting different optimized strategies in laser delivery, scanning protocol, and acoustic detection. Among all the improvements, scanning protocol is the key of the strategy for PAM miniaturization. In this section, we will discuss handheld and semi-handheld PAM based on several novel scanning devices [[100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115]]. PAMs scanning with galvanometer scanners adopting two scanning mechanisms: raster scanning and rotatory scanning, have been discussed firstly. Raster-scanning-based PAMs have realized handheld photoacoustic imaging of animal and human [[100], [101], [102]]. In contrast, rotatory-scanning-based PAMs enables the development of semi-handheld PAMs with a large FOV, a high temporal resolution, elimination of relative motion between the imaging interface and the samples for both animal and human studies [[103], [104], [105]]. In addition to galvanometer, three types of MEMS scanner based on electrothermal [[106], [107], [108], [109], [110], [111], [112]], electrostatic [113] and electromagnetic [114,[101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115]] effects have been applied in the development of handheld PAMs with ultracompact designs.
4.1. Scanning with galvanometer scanner
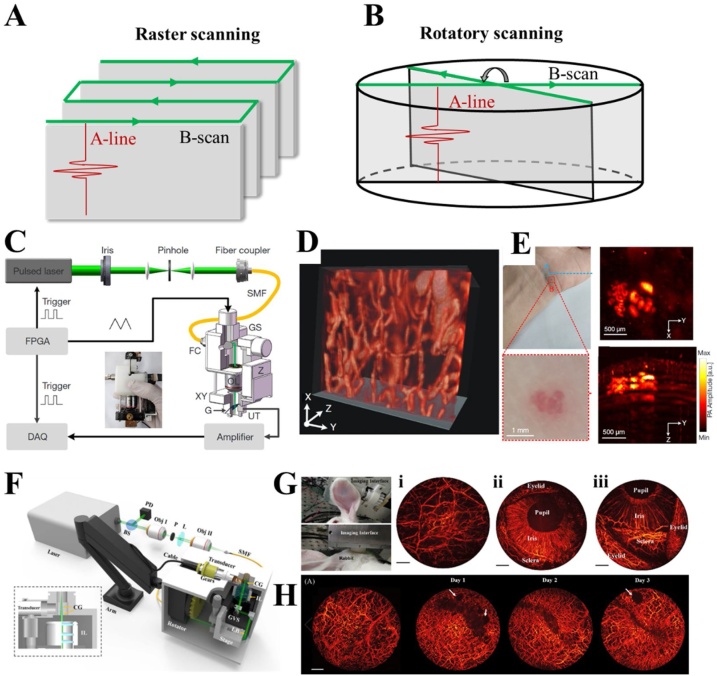
By utilizing 2D galvanometer scanners, there are two major scanning mechanisms: raster scanning mechanism (Fig. 5(A)) and rotatory scanning mechanism (Fig. 5(B)). In PAM, a laser-induced 1D depth-resolved signal (A-line) will be captured by a transducer at each point of the scanning path. Then, a 2D cross-sectional image (B-scan) can be obtained by 1D scanning of the imaging interface or the sample. Subsequently, 3D images can be finally formed by stacking multiple B-scan slices. Therefore, a 2D motorized stage is required in conventional PAMs. Unfortunately, the fast and accurate 2D mechanical scanning is time consuming and costly. In addition, there exists relative motion between the imaging probe and the samples [99]. Utilizing 2D galvanometers or MEMS scanners can realize pure optical raster scanning and has the potential to overcome these limitations [116,117]. However, this method will sacrifice part of sensitivity and SNR due to the use of flat transducers serving as acoustic detectors. A PAMs employed water-immersion galvanometer or MEMS scanner combing with focused UST to scan laser and acoustic focus. However, the damping of the water will limit the scanning speed. Rotatory scanning mechanism proposed by Xi et al. has the potential to solve these problems [118]. As shown in Fig. 5(B), each scanning point represents a depth-resolved signal (red line). When the optical focus scans along the optical scanning trace (green line), PA signals are generated along this line and detected by a cylindrically focused transducer to form a B-scan image. To realize planar scanning and 3D imaging, the optical scanning trace rotates 180° around the center of the imaging domain at a given angle step. Meanwhile, the UST synchronously rotates to achieve the overlap with the corresponding optical scanning trace. Compared with the raster scanning mechanism, rotatory scanning has a better balance between speed and sensitivity, a lager FOV (as shown in Table 1, part 3), and is free of relative motion between the imaging probe and the samples.
Fig. 5.
Handheld and semi-handheld PAM systems and the corresponding results. (A) The schematic of raster (A) and rotatory (B) scanning mechanisms. A-line (red): 1D depth-resolved signal. B-scan (gray): a cross-sectional image consisting of serial A-lines along the optical scanning path (green line). (C) The system configuration of a handheld photoacoustic microscope employing the raster scanning mechanism with a fast 2D galvanometer scanner [102]. SMF, single mode fiber. GS, galvanometer scanner. FC, fiber collimator. OL, objective lens. Z, Z-direction stage. XY, XY direction stage. G, glass. UT, ultrasonic transducer. DAQ, data acquisition, FPGA, field-programmable gate array. (D) The volumetric rendering of subcutaneous blood vessels of a human lower lip acquired by the handheld PAM [102]. (E) In vivo imaging of subcutaneous vasculatures of a human wrist. MAPs of normal skin (upper) and vascular nevus (lower) indicated by the blue and red boxes in the photograph are shown respectively [102]. (F) The schematic of a rotatory-scanning semi-handheld OR-PAM system combing with a universal arm [104]. BS, beam splitter. PD, photodiode. Obj, objective. P, pinhole. L, convex lens. SMF, single mode fiber; CG, cover glass; IL, imaging lens; GVS, galvanometer scanner; LB, laser beam. (G) A photograph of in vivo imaging experiments of a rabbit and the corresponding MAPs of ear (i) and eye (ii and iii) [104]. (H) In vivo oral imaging of a volunteer and recovery progress of an oral ulcer in the lip. Scale bar, 1 mm [104]. Reprinted with permission from Ref. [102,104]. (For interpretation of the references to colour in the Figure, the reader is referred to the web version of this article).
4.1.1. Raster-scanning-based PAM
Based on the raster scanning mechanism, several miniaturized imaging prototypes have been developed [[100], [101], [102]]. Wang et al. developed a handheld AR-PAM system consisting of a 25 MHz transducer, a two-dimensional translation stage, and laser delivery fiber bundles [101]. A Q-switched pulsed laser with a wavelength of 532 nm and a pulsed repetition rate of 10 Hz was employed to induce PA signals in melanoma. However, due to the relatively low spatial resolution (230 μm in lateral and 59 μm in axial), it is more appropriate to be classified as mesoscopy rather than microscopy (Table 1, part 3). Zemp et al., for the first time, developed a handheld real-time optical resolution photoacoustic microscope (HH-ORPAM) [100]. This design has successfully realized a handheld probe with a footprint of 4 cm × 6 cm and a weight of ∼500 g. In addition, HH-ORPAM employs a high-speed optical raster scanning by utilizing an image-guided fiber bundle (∼800 μm) consisting of 30,000 individual single mode fibers, a pulsed laser with a tunable repetition rate of up to 600 kHz, and a 2D galvanometer scanning in the proximal port of the fiber bundle. It is exciting that this HH-OR-PAM features integration and miniaturization. However, the use of fiber bundles is costly and suffers from a limited FOV and spatial resolution.
Yang et al. built a handheld photoacoustic microscope with an adjustable light focus [102]. Fig. 5(C) shows the detailed devices used in the probe, including a fiber collimator, a fast 2D galvanometer scanner, a plan achromatic objective, a XYZ-direction stages, transparent glass sheet tilted with an angle of 45° and a flat responsive UST (effective aperture: 5 mm; center frequency: 15 MHz). In the design, the fast 2D galvanometer scanner allows the raster scanning of light spot with a B-scan image speed of 25 frames per second, and the adjustment stage optimizes the optical focus to achieve the best imaging performance. The probe is able to detect subcutaneous micro-vessels in human skin, with a fast image speed, a high spatial resolution and a deep penetration depth. In detail, it takes 16 s to obtain a 2 × 2 mm2 MAP image, with a lateral resolution of ∼8.9 μm and an imaging depth of ∼2.4 mm. Fig. 5(D) and (E) present a volumetric rendering of a human lower lip and in vivo imaging of subcutaneous blood vessels of a human wrist, respectively. In the photograph, the normal skin area (blue dash box: A) and a vascular nevus area (red dash box: B) are marked. The subsequent MAP images display subcutaneous vascular in area A and morphological information in area B. Several post-processed MAP images of area B with side-view, depth-coded and edge-extracted projection show the detail information of the nevus.
4.1.2. Rotatory-scanning-based PAM
In raster-scanning-based PAM, a spherically focused UST is usually used to obtain the best SNR. To achieve three-dimensional imaging, a motorized translation stage is required to scan the imaging probe. The scanning process introduces relative motion between the imaging probe and the sample. In order to eliminate the relative motion and reduce the volume size, it is common to use flat responsive transducers instead of the spherically focused transducer and scan the laser beam with a two-dimensional galvanometer. This scanning mechanism is suffering from a low SNR [[103], [104], [105]].
Xi et al. developed the first portable/semi-handheld OR-PAM system based on a rotatory scanning mechanism [103,104]. Fig. 5(F) shows the schematic of the system, which employed a high repetition ratio Q-switched pulsed laser (up to 20,000 Hz), a fast 2D galvanometric scanner, and a scan lens to realize the fast-optical rotatory scanning. In detection, a motorized rotator equipped with a customized accelerating gear group was used to rotate a cylindrically focused transducer (center frequency: 15 MHz; aperture size: 12.7 mm, bandwidth: 75 %) to achieve confocal configuration of the optical/acoustic foci. This approach provides a large FOV of up to 8.4 mm in diameter and a depth of field (DOF) of 1.5 mm with a lateral resolution of 10.4 μm and an axial resolution of 90 μm. Compared with the traditional raster-scanning-based OR-PAM systems, the portable design based-on rotatory scanning can realize semi-automatic adjustment of placement for various image positions, with subjects in a comfortable posture. Furthermore, it also capable of multi-scale imaging of small, middle size animals and humans, representing the flexibility for different scenarios. Fig. 5(G) and (H) show the photoacoustic imaging results of a rabbit ear, rabbit eyes, and human oral lips. It is the first time for OR-PAM to track the recovery progress of an oral ulcer. All of these results demonstrate the universal applications of this approach. In addition to portable OR-PAM, Xi et al. further developed a dual modality system integrating the portable OR-PAM and a rotatory scanning spectral-domain OCT [105]. OR-PAM and spectral-domain OCT provide hemoglobin and structural alternations of an ulcer in human lip, respectively, demonstrating the clinical potential of oral inspection.
4.2. Scanning with MEMS scanner
Although the use of galvanometer scanners is able to partially reduce the volume of OR-PAMs, the size of existing galvanometer scanners prevents the further miniaturization, limiting the applications of handheld PAM. With the rapid development of MEMS devices, there are three major types of MESM scanners used to achieve further optimization of handheld OR-PAMs: electromagnetic, electrostatic and electrothermal scanners [48]. Electromagnetic MEMS scanners are usually fabricated by biaxially-oriented polyethylene (BOPET) film and soft lithography of polydimethylsiloxane (PDMS) [[119], [120], [121]]. The MEMS mirror is actuated by controlled electromagnetic forces induced by four pairs of Neodymium permanent magnets and homemade electromagnets. Different from electromagnetic MEMS, electrostatic MEMS scanners are usually fabricated with single-crystal silicon wafers. Four electrostatic bidirectional rotators driven by a steady-state analog actuation voltage result in a steady-stage analog angle of rotation of the MEMS mirror in the center [113]. The electrothermal MEMS scanner is mainly driven by four electrothermal bimorph-based actuators fabricated from a lateral-shift-free large-vertical-displacement (LSF-LVD) design [106,109,122]. Compared to the electromagnetic and electrostatic MEMS, electrothermal MEMS owns the merits of compact size, low cost, and large deflection angles, and low driven voltage, which is more suitable to develop imaging device for clinical applications.
4.2.1. Electrothermal MEMS scanners
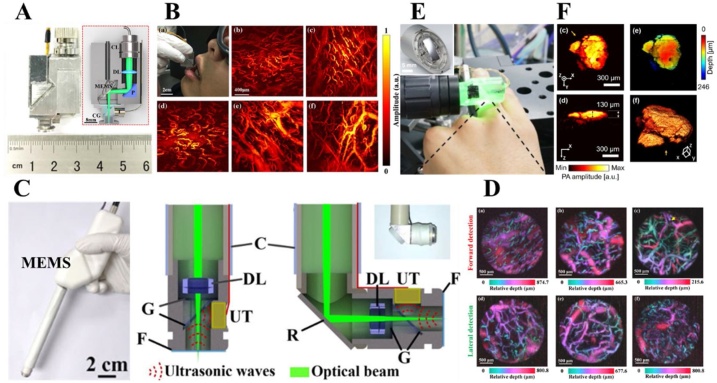
MEMS scanner was firstly implemented in PAI by Jiang et al. [123]. An electrothermal MEMS scanner was employed to scan the laser beam across a ring-shaped polyvinylidene fluoride (PVDF) UST. Further optimization studies were carried out by employing customized piezoelectric transducer [124], multi-modality system integrating diffuse optical tomography [125], human skin imaging and tumor visualization. Then, Jiang et al. and Xi et al. developed a series of photoacoustic endoscopy (PAE) based on the electrothermal MEMS scanners [126,127].
Although the volume of the handheld PAM is slightly bigger than PAE, it has better performance in terms of both spatial resolution and FOV. Xi et al. integrated all optical, acoustic, and mechanical components into a cubic probe with a volume of 22 × 30 × 13 mm3 and a weight of 20 g (Fig. 6(A)), which is the lightest in Table 1[106]. There are three key components in the probe: a two-dimensional MEMS scanner, a high NA achromatic lens and a 10 MHz flat UST. The probe can provide a frame rate of 0.2 Hz, a FOV of 2 × 2 mm2, a lateral resolution of 3.8 μm and an axial resolution of 104 μm. It has been successfully applied to carry out imaging studies of the animal organs and human oral cavity. Fig. 6(B) shows the capillary loops of a human lip inside various tissues including upper lip, lower lip, pterygomandibular fold, back surface of the tongue, and gum. Subsequently, Xi et al. proposed a dual-modality handheld microscope integrating OR-PAM and OCT systems [107]. In this dual-modality imaging probe, a cold mirror was used to allow the merge of PA and OCT light beams. In addition, two adjustable fiber collimators (350−700 nm and 650−1050 nm) were used to realize co-axial alignment of the PA and OCT foci. By further optimizing the imaging system, Xi et al. developed an ultra-compact PAM probe with a weight of ∼8 g and a diameter of ∼13 mm [111]. In the probe, an optical rotatory joint and a customized electrical slip ring were integrated to delivery excitation light and transmit electrical signals. By utilizing a special design, the probe achieves a high lateral resolution of 2.25 μm, and a faster frame rate of 0.1 Hz. Due to the light weight and high resolution, the probe can be mounted on the rat head to monitor the cerebral hemodynamics.
Fig. 6.
Handheld photoacoustic microscopy based on MEMS scanners. (A) A handheld OR-PAM based on an electrothermal MEMS scanners, with an outer size of 22 30 13 mm3 and a weight of 20 g [106]. CL, collimator. DL, doublet lens. MEMS, microelectro-mechanical system. P, prism. CG, cover glass. (B) A photograph of in vivo oral imaging using the handheld OR-PAM. (a) MAP images of microvasculature in human upper lip (b), lower lip (c), pterygomandibular fold (d), back surface of the tongue (e), and gum (f) [106]. (C) The schematic of a handheld PAI pen employing an electrostatic MEMS scanner (left). Two different mode including forward detection (middle) and lateral detection (right) are presented [113]. MEMS, microelectro-mechanical system. C, casing. DL, doublet lens. G, glass. UT, ultrasonic transducer. R, reflector. F, films. (D) PA MAPs encoded with depth of the microvasculature at the different area in oral cavity including upper lip (a), lower lip (b), sublingual (c), left wall of the oral cavity (d), right wall of the oral cavity (e), and soft palate (f) [113]. (E) A photograph of in vivo imaging of a mole on a human finger with the handheld OR-PAM probe with a diameter of 17 mm and a weight of 162 g. The inset is a photography of the electromagnetic MEMS scanners [115]. (F) MAP images (c) and cross-section slices (d) of the mole encoded with optical absorption. A MAP image encoded with depth (e) and three-dimensional rendering (f) of the mole [115]. Reprinted with permission from Ref. [106,113,115].
4.2.2. Electrostatic MEMS scanner
In addition to the electrothermal MEMS, Yang et al. developed a handheld dual-view photoacoustic imaging pen with an electrostatic MEMS scanner [113]. As shown in Fig. 6(C), they integrate the electrostatic MEMS scanner and acoustic detector with a center frequency of 10 MHz into a handheld imaging pen, which is capable of performing forward and side-view imaging. The distal head of the imaging pen has a diameter of 12 mm, which can provide an FOV of 2.4 mm in diameter, an axial resolution of 137.4 μm, an imaging speed up to 0.25 Hz, and a lateral resolution of up to 18.2 μm in different modes. Benefiting from the flexible design, the adjustable detection, and the switchable image mode, the imaging pen can meet different requirements of clinical studies, making it more suitable for clinical examination of internal areas inside the human body. Fig. 6(D) presents in vivo imaging results in different regions in human oral cavity including upper lip, lower lip, sublingual, left wall of the oral cavity, right wall of the oral cavity, and soft palate. Besides, Yang et al. proposed a handheld OR-PAM laparoscopy system based on an electrothermal MEMS scanner, which is more appropriate to be designate as a PAE platform [112].
4.2.3. Electromagnetic MEMS scanner
Apart from electrothermal and electrostatic MEMS scanners, electromagnetic MEMS scanner has been widely used in PAM for its specific merit of stability of working in water. Wang et al. proposed an OR-PAM system using a 1D water immersible electromagnetic MEMS scanner [119]. In this system, a flat transducer with a center frequency of 50 MHz was equipped with an acoustic lens to enhance the sensitivity. A one-dimensional electromagnetic MEMS scanner was immersed in the water to reflect both laser pulses and acoustic waves. Hereafter, a series of OR-PAM systems have been developed based on electromagnetic MEMS scanners [[119], [120], [121],128,129]. Wang et al. reported a handheld OR-PAM device with a volume of 80 × 115 × 150 mm3 [114]. In this probe, a two-axis electromagnetic MEMS mirror was used to achieve fast raster scanning, allowing a volumetric rate of 2 Hz over an imaging domain of 2.5 mm × 2.5 mm × 0.5 mm. In order to maximize the SNR, an optical-acoustic beam combiner was used to provide the acoustic and optical coaxial alignment. This probe was successfully applied to observe vasculatures of animals and human skins.
In contrast, Kim et al. demonstrated a handheld OR-PAM with a diameter of 17 mm and a weight of 162 g (Fig. 6(E)) based on a similar water immersible two-axis electromagnetic MEMS scanner [115]. In addition, by using a 50 kHz pulsed laser, the probe can obtain PA images with a size of 700 × 700 pixels at a volumetric imaging speed of 0.05 Hz. The lateral and axial resolutions of the probe were measured as 12 μm and 30 μm, respectively. The entire system was integrated in a medical car similar to a medical US system, which is suitable for both animal studies and patient care. Fig. 6(F) shows three-dimensional imaging results of a human mole in different views.
5. Summary
In summary, we reviewed the progresses in handheld and semi-handheld PAI systems. The general idea has been discussed in three aspects: laser delivery, scanning protocol and acoustic detection. Specific imaging systems and corresponding applications have been evaluated and discussed in terms of PACT, OAMes, and PAM.
Table 1 presents a comparison of the handheld and semi-handheld PAI platforms including laser delivery, scanning protocol, acoustic detection, and clinical applications. In terms of laser delivery, optical fibers and fiber bundles are standard methods to transmit laser energy as well as in conventional optical imaging such as OCT, diffuse optical tomography, and laser speckle contrast imaging [[130], [131], [132]]. There is a subtle difference between OR-PAM and other modalities. To achieve full-field illumination, PACT always employs a high-energy laser source and deliver the laser energy using multi-mode fibers or fiber bundles [65,71,78,82,92]. For OAMes and AR-PAM, high energy multi-mode fibers or fiber bundles are also preferred to achieve a high SNR because the spatial resolution is totally determined by acoustic diffraction [20,96,101]. In contrast, the lateral resolution of OR-PAM depends on the optical diffraction limit, thus a single-mode fiber is better to generate a Gaussian beam [102,103,106,[113], [114], [115]]. Another potential solution to miniaturize the volume of PAI system is employing LD or LED [67,81,133,134]. Compared with conventional laser source used in PACT, LD and LED can provide ultracompact design of optical delivery and high pulsed repetition rate (kHz). Although several groups have reported LD/LED-based PAM systems [133], it is still not commercially available due to the low pulsed energy and poor quality of the laser beam.
In the aspect of scanning protocol, the discussion focuses on PAM. For most conventional PACTs, the scanning process has been eliminated by using US arrays, in which linear/curved-array-based PACT can only provide two-dimensional image without additional scanning of UST array [65,67,71], and the spherical/planar-array-based PACT can realize volumetric imaging with a single pulse excitation [78,81,82]. Similar to conventional PACT, OAMes employs circular scanning around the samples [49]. To extend applications of OAMes, raster-scanning-based OAMes (RSOM) has been proposed and applied in animal studies [47,95]. Furthermore, two semi-handheld RSOM systems have been applied in visualizing subcutaneous vasculature with a higher spatial resolution in deep tissue compared with PACT [20,47,49]. In PAM, galvanometer scanners and MEMS scanners have been widely used in handheld and semi-handheld systems to accelerate imaging speed and miniaturize volumetric size. Table 1 has presented a comparison of imaging speed between RSOM (70 s), AR-PAM (10 s, B-scan mode), and PAM (5 s) based on motorized translation stage and galvanometer [20,47,[101], [102], [103]]. To eliminate the relative translation between imaging interface and samples, portable OR-PAM employs a rotatory scanning mechanism instead of conventional raster scanning mechanisms by introducing a motorized rotator [103,104]. Although the SNR of portable OR-PAM is better than that of PAM based on a flat responsive UST, the volume size of imaging probe is difficult to reduce. With the development of MEMS techniques, three major MESM scanners have been introduced to replace galvanometer scanner for further miniaturization of OR-PAM [[106], [107], [108], [109], [110], [111], [112], [113], [114], [115]]. Among them, electromagnetic MEMS scanner is water-immersible, and thus allows the scanning of both optical and acoustic beam simultaneously [114,115]. However, the volume is too large compared with electrostatic and electrothermal MEMS scanners. In contrast, electrothermal MEMS scanners have the metrics of small size, low cost, large scanning view and safe driven voltage [[106], [107], [108], [109], [110], [111], [112]]. Latest study has demonstrated a water immersive electrothermal MEMS scanner which has great potential in the development of future handheld PAM [134].
Finally, in acoustic detection, various detectors including piezoelectric UST [20,47,[101], [102], [103],106,[113], [114], [115]], UST arrays with different shapes [65,67,71,78,81,82], and acoustic sensors based on optical effect [92] are proposed in handheld and semi-handheld PAI systems. In PACT, linear-, curved-, spherical-, and planar-arrays are suitable for different applications due to the advantages of compatibility with medical US, better imaging performance, high speed of volumetric imaging, and easy coupling in point-of-care testing, respectively [65,67,71,78,81,82]. OAMes usually uses a single UST with an ultrawide bandwidth range varying from 100−200 MHz. The development of ultrasound arrays with an ultrawide bandwidth is a potential solution to accelerate the imaging speed [20,47,49]. For PAM, single UST has been applied in handheld and semi-handheld systems [[101], [102], [103],106,[113], [114], [115]]. Detecting with UST array in conjunction with micro-lens array is a potential solution to improve the frame rate from several Hz to real-time imaging [135,136]. Besides, optical detectors such as Fabry–Pérot chamber [[83], [84], [85]], micro-ring resonator [86,87], and non-interference remote sensing [[137], [138], [139]] are proposed in PAI, in which Fabry–Pérot chamber has been applied in semi-handheld PACT [[90], [91], [92]]. The optical methods can provide an ultrawide acoustic bandwidth of up to 280 MHz, resulting an isotropic spatial resolution [86,87]. Furthermore, due to the optical transparency, optical detectors have specific merit of being compatible with pure optical imaging, such as OCT, OCT angiography, and fluorescence microscopy [83,86,90,91].
By using different strategies of laser delivery, scanning protocol, and acoustic detection in PACT, OAMes, and PAM, PAI reach multiscale, multispectral, and multi-parametric imaging. In general, handheld PACTs can resolve vascular in deep tissues around 1−10 cm with a spatial resolution of hundreds of microns as shown in Table 1 [65,71,78,82,92]. In addition to hemoglobin, by inducing PA signals with multiple wavelengths, PAI can resolve other chromophores such as lipid, protein, and ICG [71,78,81]. Due to the deep penetration, handheld PACT focuses on studies of imaging guided biopsy [65,67,81,82], visualization of vasculature in deep tissues [67,71,78], and imaging of vasculature in human extremities [67,78,82]. Compare to PACT, handheld and semi-handheld PAMs have a better spatial resolution around several microns in lateral. However, the penetration depth is limited to ∼ 1 mm and the FOV is restricted in ∼ 2 × 2 mm2. Due to the high resolution, poor penetration, and small imaging domain of PAM, most handheld and semi-handheld systems are applied in visualization of superficial microvasculature in human skin and oral mucosa, which are related to melanoma and oral cancer, respectively [102,103,106,[113], [114], [115]]. Apart from PACT and PAM, OAMes has compromise resolution (tens of microns) and penetration depth (below 1 cm). Hence semi-handheld RSOMs focused on vascular and microvasculature distributing from epidermis to dermis with biomarkers related to systemic sclerosis and psoriasis [20,47].
Briefly, to address the challenges of handheld and semi-handheld PAI, new optical delivery/excitation strategies, novel optical scanning protocols, and advanced acoustic detection methods have been proposed to promote clinical translation and extend the fundamental applications of PAI. As a promising technique, there are still several aspects requiring further focus in the future: 1) A true handheld/portable PAIs without any cable and optical fiber, which are normally connected to the imaging platform for light delivery and signal transmission, is preferred. On-chip LEDs and wireless transmission of signals have the potential to achieve this goal; 2) In most PAIs, scanning is time-consuming. For PACT, scanning can be eliminated by using a 2D/3D transducer array. However, in PAM, scanning is inevitably required to achieve the high spatial resolution. The use of micro-lens array may provide an opportunity to achieve parallel scanning and reduce the scanning time; 3) In detection, comparing with piezoelectric transducer, optical method is a more attractive technique. With the improvement of the robustness of existing optical detections, PAI can be further miniaturized by using optical detections instead of conventional USTs.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Acknowledgement
This study is supported by the National Natural Science Foundation of China (62022037, 61775028, 81571722, 61528401), Department of Science and Technology of Guangdong Province (2019ZT08Y191, SZBL2020090501013), Shenzhen Science and Technology Program (KQTD20190929172743294, JCYJ20200109141222892) and Startup grant from Southern University of Science and Technology.
Biographies

Qian Chen is currently a second-year doctoral student. Now, she is working as a visiting student in the Multi-Functional Optical Imaging Lab (MFOIL) at the Southern University of Science and Technology, Shenzhen, China. Her research focuses on developing novel handheld and wearable optical resolution photoacoustic microscopy systems for biological and clinical research.

Wei Qin is a graduate student in University of Electronic Science and Technology of China (UESTC). He received his B.S in fundamental science of mathematics and physics from UESTC in 2018. He has worked in research of photoacoustic imaging and optical coherence tomography.

Dr. Weizhi Qi received the B.S. degree and Ph. D. degree from the University of Electronic Science and Technology of China (UESTC), Chengdu, China in 2012 and 2018, respectively. He is a post-doctor and currently working in the Multi-Functional Optical Imaging Lab (MFOIL) at the Southern University of Science and Technology, Shenzhen, China. His research focuses on developing high speed optical resolution photoacoustic microscopy.

Prof. Lei Xi received his bachelor degree from Huazhong University of Science and Technology, Wuhan, China (2007) in optics, and finished his doctoral and post-doctoral training in the Department of Biomedical Engineering at the University of Florida, Gainesville, USA, in 2012 and 2014, respectively. Then, he worked at the University of Electronic Science and Technology of China (UESTC) from 2014 to 2018 as a professor. Now, he join the Department of Biomedical Engineering at the Southern University of Science and Technology (SUSTech). He is hosting the multifunctional optical imaging lab (MFOIL) in SUSTech and focusing on developing novel optical imaging techniques for different biomedical and clinical studies.
Appendix A
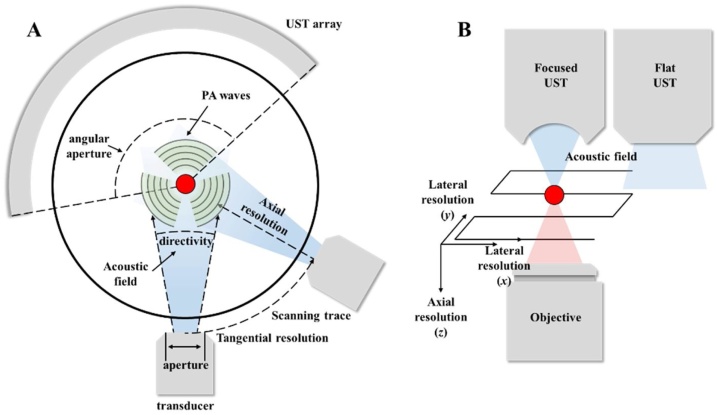
Appendix Fig. A1(A) presents the schematic of PACT to clarify several definitions in PAI. For a conventional PACT, a single element transducer scans along the circle around the sample. The tangential and axial resolutions refer to the resolution along the tangential direction of the scanning track and the axial direction of the transducer, respectively [6,7]. In general, the axial resolution is determined by the frequency of ultrasound, in which a transducer with a higher center frequency and wider bandwidth brings a better axial resolution. The tangential resolution depends on the aperture of transducer, which refers to the active detection area of the detecting element. There is a tradeoff between high tangential resolution and detecting sensitivity. A small aperture means a high tangential resolution but a poor sensitivity. Another important feature of the imaging performance is the directivity of the transducer, which refers to the acoustic radiation angle when the radiation power decreases to −6 dB of the peak power in the center of the transducer. Thus, a larger directivity also means a better acceptance angle of a transducer. For a transducer array (as shown in appendix Fig. A1(A)), the angular aperture refers to the angle coverage as shown in appendix Fig. A1(A). Since the PA pressure signal is a spherical wave, a limited view array (as shown in appendix Fig. A1(A)) will lose signals out of the view, while a full-view (360° angular aperture) array can receive all the signals in the imaging plane.
Fig. A1.
Several definitions in PAI. (A) Schematic of the conventional PACT and transducer-array-based PACT. (B) Schematic of raster-scanning-based PAM.
For raster scanning PAM or RSOM, two parameters, lateral and axial resolution, characterize the spatial resolution of imaging systems. Similar to PACT, the axial resolution in PAM and RSOM means the resolution in axial direction of the transducer. The axial resolution is primary determined by the acoustic diffraction limit, which is same as that in PACT. A transducer with a higher center frequency and wider bandwidth can bring a better axial resolution. Lateral resolution refers to the resolution in scanning directions (x, y) of light or transducer as shown in appendix Fig. A1(B). In AR-PAM and RSOM, the lateral resolution usually depends on the size of the acoustic focus. In OR-PAM, the lateral resolution depends on the size of the optical focus. In PAM, the type of transducer will affect the SNR/detection sensitivity. As shown in appendix Fig. A1(B), PAMs with a focused UST can achieve a high SNR because the response is greater at focus. On the contrary, a flat transducer has a uniform response in entire acoustic detection filed but the sensitivity is smaller.
References
- 1.Wang L.V., Hu S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science. 2012;335(6075):1458–1462. doi: 10.1126/science.1216210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paul B. Biomedical photoacoustic imaging. Interface Focus. 2011;1(4):602–631. doi: 10.1098/rsfs.2011.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L., Zhu L., Ma C., Lin L., Yao J., Wang L., Maslov K., Zhang R., Chen W., Shi J., Wang L.V. Single-impulse panoramic photoacoustic computed tomography of small-animal whole-body dynamics at high spatiotemporal resolution. Nat. Biomed. Eng. 2017;1(5):1–11. doi: 10.1038/s41551-017-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L.V., Gao L. Photoacoustic microscopy and computed tomography: from bench to bedside. Annu. Rev. Biomed. Eng. 2014;16:155–185. doi: 10.1146/annurev-bioeng-071813-104553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi J., Wong T.T.W., He Y., Li L., Zhang R., Yung C.S., Hwang J., Maslov K., Wang L.V. High-resolution, high-contrast mid-infrared imaging of fresh biological samples with ultraviolet-localized photoacoustic microscopy. Nat. Photon. 2019;13:609–615. doi: 10.1038/s41566-019-0441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pramanik M., Ku G., Wang L.V. Tangential resolution improvement in thermoacoustic and photoacoustic tomography using a negative acoustic lens. J. Biomed. Opt. 2009;14(2) doi: 10.1117/1.3103778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang S., Qin W., Guo H., Jin T., Huang N., He M., Xi L. Design and evaluation of a compound acoustic lens for photoacoustic computed tomography. Biomed. Opt. Express. 2017;8(5):2756–2765. doi: 10.1364/BOE.8.002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park J., Jeon S., Meng J., Song L., Lee J.S., Kim C. Delay-multiply-and-sum-based synthetic aperture focusing in photoacoustic microscopy. J. Biomed. Opt. 2016;21(3) doi: 10.1117/1.JBO.21.3.036010. [DOI] [PubMed] [Google Scholar]
- 9.Jo J.G., Tian C., Xu G., Sarazin J., Schiopud E., Gandikota G., Wang X.D. Photoacoustic tomography for human musculoskeletal imaging and inflammatory arthritis detection. Photoacoustics. 2018;12:82–89. doi: 10.1016/j.pacs.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin L., Hu P., Shi J.H., Appleton C.M., Maslov K., Li L., Zhang R.Y., Wang L.V. Single-breath-hold photoacoustic computed tomography of the breast. Nat. Commun. 2018;9:2352. doi: 10.1038/s41467-018-04576-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao L., Jiang H.B. Enhancing finite element-based photoacoustic tomography using total variation minimization. Appl. Opt. 2011;50(25):531–5041. doi: 10.1364/AO.50.005031. [DOI] [Google Scholar]
- 12.Hauptmann A., Lucka F., Betcke M., Huynh N., Adler J., Cox B., Paul B. Model-based learning for accelerated, limited-view 3-D photoacoustic tomography. IEEE Trans. Med. Imaging. 2018;37(6):1382–1393. doi: 10.1109/TMI.2018.2820382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park S., Lee C., Kim J., Kim C. Acoustic resolution photoacoustic microscopy. Biomed. Eng. Lett. 2014;4(3):213–222. [Google Scholar]
- 14.Hai P., Imai T., Xu S., Zhang R., Aft R.L., Zou J., Wang L.V. High-throughput, label-free, single-cell photoacoustic microscopy of intratumoral metabolic heterogeneity. Nat. Biomed. Eng. 2019;3(5):381–391. doi: 10.1038/s41551-019-0376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Z., Li Y., Chen S. Miniature probe for in vivo optical- and acoustic-resolution photoacoustic microscopy. Opt. Lett. 2018;43:1119–1122. doi: 10.1364/OL.43.001119. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz M., Omar M., Buehler A., Aguirre J., Ntziachristos V. Implications of ultrasound frequency in optoacoustic mesoscopy of the skin. IEEE Trans. Med. Imaging. 2015;34(2):672–677. doi: 10.1109/TMI.2014.2365239. [DOI] [PubMed] [Google Scholar]
- 17.Omar M., Soliman D., Gateau J., Ntziachristos V. Ultrawideband reflection-mode optoacoustic mesoscopy. Opt. Lett. 2014;39(13):3911–3914. doi: 10.1364/OL.39.003911. [DOI] [PubMed] [Google Scholar]
- 18.Stein E.W., Maslov K., Wang L.V. Noninvasive mapping of the electrically stimulated mouse brain using photoacoustic microscopy – art. Proc, Spie Int. Soc. Optic. Eng. 2008:6856. [Google Scholar]
- 19.Hai P.F., Yao J.J., Maslov K.I., Zhou Y., Wang L.V. Near-infrared optical-resolution photoacoustic microscopy. Opt. Lett. 2014;39(17):5192–5195. doi: 10.1364/OL.39.005192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aguirre J., Schwarz M., Garzorz N., Omar M., Buehler A., Eyerich K., Ntziachristos V. Precision assessment of label-free psoriasis biomarkers with ultra-broadband optoacoustic mesoscopy. Nat. Biomed. Eng. 2017;1(5):0068. doi: 10.1038/s41551-017-0068. [DOI] [Google Scholar]
- 21.Deán-Ben X.Luís, Sela G., Lauri A., Kneipp M., Ntziachristos V., Westmeyer G., Shoham S., Razansky D. Functional optoacoustic neuro-tomography for scalable whole-brain monitoring of calcium indicators. Light Sci. Appl. 2016;5(12) doi: 10.1038/lsa.2016.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia J., Wang L.V. Small-animal whole-body photoacoustic tomography: a review. IEEE Trans. Biomed. Eng. 2014;61(5):1380–1389. doi: 10.1109/TBME.2013.2283507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh C.G., Li L., Zhu L.R., Xia J., Li C.Y., Chen W.Y., Garcia-Uribe A., Maslov K.I., Wang L.V. Dry coupling for whole-body small-animal photoacoustic computed tomography. J. Biomed. Opt. 2017;22(4) doi: 10.1117/1.JBO.22.4.041017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jo J.G., Zhang H.Y., Cheney P.D., Yang X.M. Photoacoustic detection of functional responses in the motor cortex of awake behaving monkey during forelimb movement. J. Biomed. Opt. 2012;17(11) doi: 10.1117/1.JBO.17.11.110503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xi L., Jin T., Zhou J.L., Carney P.R., Jiang H.B. Hybrid photoacoustic and electrophysiological recording of neurovascular communications in freely-moving rats. Neuroimage. 2017;161:232–240. doi: 10.1016/j.neuroimage.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 26.Tang J.B., Xi L., Zhou J.L., Huang H., Zhang T., Carney P.R., Jiang H.B. Noninvasive high-speed photoacoustic tomography of cerebral hemodynamics in awake-moving rats. J. Cerebral Blood Flow Metabolism. 2015;35(8):1224–1232. doi: 10.1038/jcbfm.2015.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toi M., Asao Y., Matsumoto Y., Sekiguchi H., Yoshikawa A., Takada M., Kataoka M., Endo T., Kawaguchi-Sakita N., Kawashima M., Fakhrejahani E., Kanao S., Yamaga I., Nakayama Y., Tokiwa M., Torii M., Yagi T., Sakurai T., Togashi K., Shiina T. Visualization of tumor-related blood vessels in human breast by photoacoustic imaging system with a hemispherical detector array. Sci. Rep. 2017;7 doi: 10.1038/srep41970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeh C., Liang J., Zhou Y., Hu S., Sohn R.E., Arbeit J.M., Wang L.V. Photoacoustic microscopy of arteriovenous shunts and blood diffusion in early-stage tumors. J. Biomed. Opt. 2016;21(2) doi: 10.1117/1.JBO.21.2.020501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen, Phuc V., Paulus, Yannis M. Photoacoustic ophthalmoscopy: principle, application, and future directions. J. Imaging. 2018;4(12) doi: 10.3390/jimaging4120149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen V., Li Y., Aaberg M., Zhang W., Wang X.D., Paulus Y.M. In vivo 3D imaging of retinal neovascularization using multimodal photoacoustic microscopy and optical coherence tomography imaging. J. Imaging. 2018;4(12) doi: 10.3390/jimaging4120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knieling F., Neufert M.F., Waldner M.J. Multispectral optoacoustic tomography for assessment of Crohn’s disease activity. N. Engl. J. Med. 2017;376(13):1292–1294. doi: 10.1056/NEJMc1612455. [DOI] [PubMed] [Google Scholar]
- 32.Qin W., Qi W.Z., Xi L. Quantitative investigation of vascular response to mesenteric venous thrombosis using large‐field‐of‐view photoacoustic microscopy. J. Biophotonics. 2019;12(12) doi: 10.1002/jbio.201900198. (1) [DOI] [PubMed] [Google Scholar]
- 33.Li Y., Chen Z.P. Multimodal intravascular photoacoustic and ultrasound imaging. Biomed. Eng. Lett. 2018;8(2):193–201. doi: 10.1007/s13534-018-0061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iskander-Rizk S., Wu M., Springeling G., van Beusekom H.M.M., Mastik F., Hekkert M.T., Beurskens R.H.S.H., Hoogendoorn A., Hartman E.M.J., van der Steen A.F.W., Wentzel J.J., van Soest G. In vivo intravascular photoacoustic imaging of lipid plaque in coronary atherosclerosis. EuroIntervention: J. EuroPCR collaboration Working Group Interventional Cardiology European Society Cardiology. 2019;15(5):452–+. doi: 10.4244/EIJ-D-19-00318. [DOI] [PubMed] [Google Scholar]
- 35.Li X.Q., Heldermon C.D., Yao L., Xi L., Jiang H.B. High resolution functional photoacoustic tomography of breast cancer. Med. Phys. 2015;42(9):5321–5328. doi: 10.1118/1.4928598. [DOI] [PubMed] [Google Scholar]
- 36.Nyayapathi N., Lim R., Zhang H.J., Zeng W.H., Wang Y.H., Tiao M., Oh K.W., Fan X.C., Bonaccio E., Takabe K., Xia J. Dual scan mammoscope (DSM)—a new portable photoacoustic breast imaging system with scanning in Craniocaudal Plane. IEEE. Trans. Biomed. Eng. 2020;67(5):1321–1327. doi: 10.1109/TBME.2019.2936088. [DOI] [PubMed] [Google Scholar]
- 37.Nyayapathi N., Lim R., Zhang H., Zheng W., Wang Y., Tiao M., Oh K.W., Fan X.C., Bonaccio E., Takabe K., Xia J. Dual Scan Mammoscope (DSM)-A New Portable Photoacoustic Breast Imaging System With Scanning in Craniocaudal Plane. IEEE Trans. Biomed. Eng. 2020;67(5):1321–1327. doi: 10.1109/TBME.2019.2936088. [DOI] [PubMed] [Google Scholar]
- 38.Zhang P.F., Li L., Lin L., Hu P., Shi J.H., He Y., Zhu L.R., Zhou Y., Wang L.V. High-resolution deep functional imaging of the whole mouse brain by photoacoustic computed tomography in vivo. J. Biophotonics. 2017;11(1) doi: 10.1002/jbio.201700024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu M., Maurer B., Hermann B., Zabihian B., Sandrian M.G., Unterhuber A., Baumann B., Zhang E.Z., Beard P.C., Weninger W.J., Drexler W. Dual modality optical coherence and whole-body photoacoustic tomography imaging of chick embryos in multiple development stages. Biomed. Opt. Express. 2014;5(9):3150–3159. doi: 10.1364/BOE.5.003150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mason W., Schellenberg, Heather K. Hand-held optoacoustic imaging: a review. Photoacoustics. 2018;11:14–27. doi: 10.1016/j.pacs.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi W., Park E.Y., Jeon S., Kim C. Clinical photoacoustic imaging platforms. Biomed. Eng. Lett. 2018;8(2):139–155. doi: 10.1007/s13534-018-0062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia W., Nikitichev D.I., Mari J.M., West S.J., Ourselin S., Beard P.C., Desjardins A.E. An interventional multispectral photoacoustic imaging platform for the guidance of minimally invasive procedures, European Conference on Biomedical Optics. Optic. Soc. Am. 2015 doi: 10.1117/12.2183798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dima A., Burton N.C., Ntziachristos V. Multispectral optoacoustic tomography at 64, 128, and 256 channels. J. Biomed. Opt. 2014;19(3) doi: 10.1117/1.JBO.19.3.036021. [DOI] [PubMed] [Google Scholar]
- 44.Yuan Y., Yang S.H., Xing D. Optical-resolution photoacoustic microscopy based on two-dimensional scanning galvanometer. Appl. Phys. Lett. 2012;100(2) doi: 10.1063/1.3675907. [DOI] [Google Scholar]
- 45.Qi W.Z., Jin T., Rong J., Jiang H.B., Xi L. Inverted multiscale optical resolution photoacoustic microscopy. J. Biophotonics. 2017;10(12):1580–1585. doi: 10.1002/jbio.201600246. [DOI] [PubMed] [Google Scholar]
- 46.Park K., Kim J.Y., Lee C., Jeon S., Lim G., Kim C. Handheld photoacoustic microscopy probe. Sci. Rep. 2017;7(1):1–15. doi: 10.1038/s41598-017-13224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aguirre J., Hindelang B., Bereznhoi A., Darsow U., Lauffer F., Eyerich K., Biedermann T., Ntziachristos V. Assessing nailfold microvascular structure with ultra-wideband raster-scan optoacoustic mesoscopy. Photoacoustics. 2018;10:31–37. doi: 10.1016/j.pacs.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee C., Kim J.Y., Kim C. Recent progress on photoacoustic imaging enhanced with microelectromechanical systems (MEMS) technologies. Micromachines. 2018;9(11):584. doi: 10.3390/mi9110584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Omar M., Aguirre J., Ntziachristos V. Optoacoustic mesoscopy for biomedicine. Nat. Biomed. Eng. 2019;3(5):354–370. doi: 10.1038/s41551-019-0377-4. [DOI] [PubMed] [Google Scholar]
- 50.Zhang P.F., Li L., Shi J.H., Wang L.V. In vivo super resolution photoacoustic computed tomography by localization of single dyed droplets. Light Sci. Appl. 2019;8(1):1–9. doi: 10.1038/s41377-019-0147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X.D., Xie X.Y., Ku G., Wang L.V., Stiica G. Noninvasive imaging of hemoglobin concentration and oxygenation in the rat brain using high-resolution photoacoustic tomography. J. Biomed. Optics. 2006;11(2) doi: 10.1117/1.2192804. [DOI] [PubMed] [Google Scholar]
- 52.Huang N., He M., Shi H.S., Zhao Y., Lu M., Zou X.B., Yao L., Jiang H.B. Curved-array-based multispectral photoacoustic imaging of human finger joints. IEEE Trans. Biomed. Eng. 2017;65(7):1452–1459. doi: 10.1109/TBME.2017.2758905. [DOI] [PubMed] [Google Scholar]
- 53.Lin L., Hu P., Shi J.H., Appleton C.M., Maslov K., Li L., Zhang R.Y., Wang L.V. Single-breath-hold photoacoustic computed tomography of the breast. Nat. Commun. 2018;9(1):2352. doi: 10.1038/s41467-018-04576-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo H., Wang Q., Qi W.Z., Sun X., Ke B.W., Xi L. Assessing the development and treatment of rheumatoid arthritis using multiparametric photoacoustic and ultrasound imaging. J. Biophotonics. 2019;12(11) doi: 10.1002/jbio.201900127. [DOI] [PubMed] [Google Scholar]
- 55.Nishiyama M., Namita T., Kondo K., Yamakawa M., Shiina T. Ring-array photoacoustic tomography for imaging human finger vasculature. J. Biomed. Opt. 2019;24(9) doi: 10.1117/1.JBO.24.9.096005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wray P., Lin L., Hu P., Wang L.V. Photoacoustic computed tomography of human extremities. J. Biomed. Opt. 2019;24(2) doi: 10.1117/1.JBO.24.2.026003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia-Uribe A., Erpelding T.N., Krumholz A., Ke X.H., Maslov K., Appleton C., Margenthaler J.A., Wang L.V. Dual-modality photoacoustic and ultrasound imaging system for noninvasive sentinel lymph node detection in patients with breast cancer. Sci. Rep. 2015;5:15748. doi: 10.1038/srep15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou Y., Tripathi S.V., Rosman I., Ma J., Hai P., Linette G.P., Council M.L., Fields R.C., Wang L.V., Cornelius L.A. Noninvasive determination of melanoma depth using a handheld photoacoustic probe. J Invest Dermatol. 2017;137(6):1370–1372. doi: 10.1016/j.jid.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sivasubramanian K., Periyasamy V., Pramanik M. Non‐invasive sentinel lymph node mapping and needle guidance using clinical handheld photoacoustic imaging system in small animal. J. Biophotonics. 2018;11(1) doi: 10.1002/jbio.201700061. [DOI] [PubMed] [Google Scholar]
- 60.Irisawa K., Hirota K., Hashimoto A., Murakoshi D., Ishii H., Tada H., Wada T., Hayakawa T., Azuma R., Otani N., Itoh K., Ishihara M. Photoacoustic imaging system for peripheral small-vessel imaging based on clinical ultrasound technology. Proc. SPIE 9708, Photons Plus Ultrasound: Imaging and Sensing. 2016 doi: 10.1117/12.2211352. 970807 (15 March 2016) [DOI] [Google Scholar]
- 61.Kim J., Park S., Jung Y., Chang S., Park J., Zhang Y.M., Lovell J.F., Kim C. Programmable real-time clinical photoacoustic and ultrasound imaging system. Sci. Rep. 2016;6:35137. doi: 10.1038/srep35137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang M., Zhao L.Y., He X.J., Su N., Zhao C.Y., Tang H.W., Hong T., Li W.B., Yang F., Lin L., Zhang B., Zhang R. Photoacoustic/ultrasound dual imaging of human thyroid cancers: an initial clinical study. Biomed. Opt. express. 2017;8(7) doi: 10.1364/BOE.8.003449. 3499-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao Y.J., Yu SH Tao B., Gao F. Adjustable handheld probe design for photoacoustic imaging: experimental validation. 2019 41st Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC); IEEE,; 2019. pp. 7119–7122. [DOI] [PubMed] [Google Scholar]
- 64.Jo J.G., Xu G., Cao M., Marquardt A., Francis S., Gandikota G., Wang X.D. A functional study of human inflammatory arthritis using photoacoustic imaging. Sci. Rep. 2017;7:15026. doi: 10.1038/s41598-017-15147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li M.C., Liu C.B., Gong X.J., Zeng R.Q., Bai Y.Y., Xing M.Y., Du X.M., Liu X.Y., Zeng J., Lin R.Q. Linear array-based real-time photoacoustic imaging system with a compact coaxial excitation handheld probe for noninvasive sentinel lymph node mapping. Biomed. Opt. Express. 2018;9(4):1408–1422. doi: 10.1364/BOE.9.001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Daoudi K., Van den Berg P.J., Rabot O., Kohl A., Tisserand S., Brands P., Steenbergen W. Handheld probe integrating laser diode and ultrasound transducer array for ultrasound/photoacoustic dual modality imaging. Opt. Express. 2014;22(21):26365–26374. doi: 10.1364/OE.22.026365. [DOI] [PubMed] [Google Scholar]
- 67.Xia W.F., Singh M.K.A., Maneas E., Sato N., Shigeta Y., Agano T., Ourselin S., West S.J., Desjardins A.E. Handheld real-time LED-Based photoacoustic and ultrasound imaging system for accurate visualization of clinical metal needles and superficial vasculature to guide minimally invasive procedures. Sensors. 2018;18(5):1394. doi: 10.3390/s18051394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buehler A., Kacprowicz M., Taruttis A., Ntziachristos V. Real-time handheld multispectral optoacoustic imaging. Opt. Lett. 2013;38(9):1404–1406. doi: 10.1364/OL.38.001404. [DOI] [PubMed] [Google Scholar]
- 69.Diot G., Dima A., Ntziachristos V. Multispectral opto-acoustic tomography of exercised muscle oxygenation. Opt. Lett. 2015;40(7):1496–1499. doi: 10.1364/OL.40.001496. [DOI] [PubMed] [Google Scholar]
- 70.Dima A., Ntziachristos V. In-vivo handheld optoacoustic tomography of the human thyroid. Photoacoustics. 2016;4(2):65–69. doi: 10.1016/j.pacs.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taruttis A., Timmermans A.C., Wouters P.C., Kacprowicz M., van Dam G.M., Ntziachristos V. Optoacoustic imaging of human vasculature: feasibility by using a handheld probe. Radiology. 2016;281(1):256–263. doi: 10.1148/radiol.2016152160. [DOI] [PubMed] [Google Scholar]
- 72.Dean-Ben X.L., Mercep E., Razansky D. Hybrid-array-based optoacoustic and ultrasound (OPUS) imaging of biological tissues. Appl Phys Lett. 2017;110(20):68–73. doi: 10.1063/1.4983462. [DOI] [Google Scholar]
- 73.Neuschmelting V., Burton N.C., Lockau H., Urich A., Harmsen S., Ntziachristos V., Kircher M.F. Performance of a Multispectral Optoacoustic Tomography (MSOT) System equipped with 2D vs. 3D Handheld Probes for Potential Clinical Translation. Photoacoustics. 2016;4(1):1–10. doi: 10.1016/j.pacs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deán-Ben X.L., Razansky D. Portable spherical array probe for volumetric real-time optoacoustic imaging at centimeter-scale depths. Opt. Express. 2013;21(23):28062–28071. doi: 10.1364/OE.21.028062. [DOI] [PubMed] [Google Scholar]
- 75.Deán-Ben, Luís Xosé, Razansky D. Functional optoacoustic human angiography with handheld video rate three dimensional scanner. Photoacoustics. 2013;1(3–4):68–73. doi: 10.1016/j.pacs.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deán-Ben X.L., Razansky D. Adding fifth dimension to optoacoustic imaging: volumetric time-resolved spectrally enriched tomography. Light Sci. Appl. 2014;3(1):e137. doi: 10.1038/lsa.2014.18. [DOI] [Google Scholar]
- 77.Deán-Ben X.L., Fehm T.F., Gostic M., Razansky D. Volumetric hand-held optoacoustic angiography as a tool for real-time screening of dense breast. J. Biophotonics. 2016;9(3):253–259. doi: 10.1002/jbio.201500008. [DOI] [PubMed] [Google Scholar]
- 78.Ivankovic I., Merčep E., Schmedt C.G., Dean-Ben X.L., Razansky D. Real-time volumetric assessment of the human carotid artery: handheld multispectral optoacoustic tomography. Radiology. 2019;291(1):44–49. doi: 10.1148/radiol.2019181325. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y., Erpelding T.N., Jankovic L., Guo Z.J., Robert J.L., David G., Wang L.V. In vivo three-dimensional photoacoustic imaging based on a clinical matrix array ultrasound probe. J. Biomed Opt. 2012;17(6) doi: 10.1117/1.JBO.17.6.061208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu S.Y., Feng X.H., Jin H.R., Zhang R.C., Luo Y.Q., Zeng Z.S., Gao F., Zheng Y.Z. Handheld photoacoustic imager for theranostics in 3D. IEEE Trans. Med. Imaging. 2019;38(9):2037–2046. doi: 10.1109/TMI.2019.2900656. [DOI] [PubMed] [Google Scholar]
- 81.Liu S.Y., Tang K., Feng X.H., Jin H.R., Gao F., Zheng Y.J. Toward Wearable Healthcare: A Miniaturized 3D Imager With Coherent Frequency-Domain Photoacoustics. IEEE T BIOMED CIRS S. 2019;13(6):1417–1424. doi: 10.1109/TBCAS.2019. 2940243. [DOI] [PubMed] [Google Scholar]
- 82.Liu S.Y., Song W.T., Liao X.Q., Kin T.T.H., Zheng Y.J. Development of a handheld volumetric photoacoustic imaging system with a central-holed 2D matrix aperture. IEEE T Biomed. Eng. 2020;67(9):2482–2489. doi: 10.1109/TBME.2019.2963464. [DOI] [PubMed] [Google Scholar]
- 83.Zhang E.Z., Povazay B., Laufer J., Alex A., Hofer B., Pedley B., Glittenberg C., Treeby B., Cox B., Beard P., Drexler W. Multimodal photoacoustic and optical coherence tomography scanner using an all optical detection scheme for 3D morphological skin imaging. Biomed. Opt. Express. 2018;2(8):2202–2215. doi: 10.1364/BOE.2.002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guggenheim J.A., Li J., Allen T.J., Colchester R.J., Noimark S., Ogunlade O., Parkin I.P., Papakonstantinou I., Desjardins A.E., Zhang E.Z., Beard P.C. Ultrasensitive plano-concave optical microresonators for ultrasound sensing. Nat. Photon. 2017;11(11):714–719. doi: 10.1038/s41566-017-0027-x. [DOI] [Google Scholar]
- 85.Preisser S., Rohringer W., Liu M.Y., Kollmann C., Zotter S., Fischer B., Drexler W. All-optical highly sensitive akinetic sensor for ultrasound detection and photoacoustic imaging. Biomed. Opt. Express. 2016;7(10):4171–4186. doi: 10.1364/BOE.7.004171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dong B.Q., Li H., Zhang Z., Zhang K., Chen S.Y., Sun C., Zhang H.F. Isometric multimodal photoacoustic microscopy based on optically transparent micro-ring ultrasonic detection. Optica. 2015;2(2):169–176. doi: 10.1364/OPTICA.2.000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li H., Dong B.Q., Zhang X., Shu X., Chen X.F., Hai R.H., Czaplewski D.A., Zhang H.F., Sun C. Disposable ultrasound-sensing chronic cranial window by soft nanoimprinting lithography. Nat. Commun. 2019;10(1):4277. doi: 10.1038/s41467-019-12178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang E.Z., Povazay Laufer, Alex Hofer, Pedley Glittenberg C., Treeby B., Cox B., Beard P., Drexler W. Multimodal photoacoustic and optical coherence tomography scanner using an all optical detection scheme for 3D morphological skin imaging. Biomed. Opt. Express. 2011;2(8):2202–2215. doi: 10.1364/BOE.2.002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu M.Y., Maurer B., Hermann B., Zabihian B., Sandrian M.G., Unterhuber A., Baumann B., Zhang E.Z., Beard P.C., Weninger W.J., Drexler W. Dual modality optical coherence and whole-body photoacoustic tomography imaging of chick embryos in multiple development stages. Biomed. Opt. Express. 2014;5(9):3150–3159. doi: 10.1364/BOE.5.003150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Z., Rank E., Meiburger K.M., Sinz C., Hodul A., Zhang E., Hoover E., Minneman M., Ensher J., Beard P.C., Kittler H., Leitgeb R.A., Drexler W., Liu M. Non-invasive multimodal optical coherence and photoacoustic tomography for human skin imaging. Sci. Rep. 2017;7(1):1–11. doi: 10.1038/s41598-017-18331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu M.Y., Chen Z., Zabihian B., Sinz C., Zhang E., Beard P.C., Ginner L., Hoover E., Minneman M.P., Leitgeb R.A., Kittler H., Drexler W. Combined multi-modal photoacoustic tomography, optical coherence tomography (OCT) and OCT angiography system with an articulated probe for in vivo human skin structure and vasculature imaging. Biomed. Opt. Express. 2016;7(9):3390–3402. doi: 10.1364/BOE.7.003390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Plumb A.A., Huynh N.T., Guggenheim J., Zhang E., Beard P. Rapid volumetric photoacoustic tomographic imaging with a Fabry-Perot ultrasound sensor depicts peripheral arteries and microvascular vasomotor responses to thermal stimuli. Eur. Radiol. 2018;28(3):1037–1045. doi: 10.1007/s00330-017-5080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Omar M., Gateau J., Ntziachristos V. Raster-scan optoacoustic mesoscopy in the 25–125MHz range. Opt. Lett. 2013;38(14):2472–2474. doi: 10.1364/OL.38.002472. [DOI] [PubMed] [Google Scholar]
- 94.Li C., Wang L.V. Photoacoustic tomography of the mouse cerebral cortex with a high-numerical-aperture-based virtual point detector. J. Biomed. Opt. 2009;14(2) doi: 10.1117/1.3122365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Omar M., Schwarz M., Soliman D., Symvoulidis P., Ntziachristos V. Pushing the optical imaging limits of Cancer with multi-frequency-Band raster-scan optoacoustic mesoscopy (RSOM) Neoplasia. 2015;17(2):208–214. doi: 10.1016/j.neo.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haedicke K., Agemy L., Omar M., Berezhnoi A., Roberts S., Longo-Machado C., Skubal M., Nagar K., Hsu H.T., Kim K. High-resolution optoacoustic imaging of tissue responses to vascular-targeted therapies. Nat. Biomed. Eng. 2020;4(3):286–297. doi: 10.1038/s41551-020-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jeon S., Kim J., Lee D., Baik J.W., Kim C. Review on practical photoacoustic microscopy. Photoacoustics. 2019;15 doi: 10.1016/j.pacs.2019.100141. UNSP 100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yao J, Wang LV Photoacoustic microscopy. Laser Photonics Rev. 2013;7(5):758–778. doi: 10.1002/lpor.201200060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen Q., Jin T., Qi W.Z., Mo X.M., Xi L. Label-free photoacoustic imaging of the carido-cerebrovascular development in the embryonic zebrafish. Biomed. Opt. Express. 2017;8(4):2359–2367. doi: 10.1364/BOE.8.002359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hajireza P., Shi W., Zemp R.J. Real-time handheld optical-resolution photoacoustic microscopy. Opt. Express. 2011;19(21):20097–20102. doi: 10.1364/OE.19.020097. [DOI] [PubMed] [Google Scholar]
- 101.Zhou Y., Xing W.X., Maslov K.I., Cornelius L.A., Wang L.V. Handheld photoacoustic microscopy to detect melanoma depth in vivo. Opt. Lett. 2014;39(16):4731–4734. doi: 10.1364/OL.39.004731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang W.Y., Ma H.G., Cheng Z.W., Wang Z.Y., Zhang L., Yang S.H. Miniaturized photoacoustic probe for in vivo imaging of subcutaneous microvessels within human skin. Quant. Imaging Med. Surg. 2019;9(5):807–814. doi: 10.21037/qims.2019.05.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jin T., Guo H., Yao L., Xie H.K., Jiang H.B., Xi L. Portable optical‐resolution photoacoustic microscopy for volumetric imaging of multiscale organisms. J. Biophotonics. 2018;11(4) doi: 10.1002/jbio.201700250. [DOI] [PubMed] [Google Scholar]
- 104.Jin T., Guo H., Jiang H.K., Ke B.W., Xi L. Portable optical resolution photoacoustic microscopy (pORPAM) for human oral imaging. Opt. Lett. 2017;42(21):4434–4437. doi: 10.1364/OL.42.004434. [DOI] [PubMed] [Google Scholar]
- 105.Qin W., Qi W.Z., Jin T., Xi L. In vivo oral imaging with integrated portable photoacoustic microscopy and optical coherence tomography. Appl. Phys. Lett. 2017;111(26) doi: 10.1063/1.5006234. [DOI] [Google Scholar]
- 106.Chen Q., Guo H., Jin T., Qi W.Z., Xie H.K., Xi L. Ultracompact high-resolution photoacoustic microscopy. Opt. Lett. 2018;7(43):1615–1618. doi: 10.1364/OL.43.001615. [DOI] [PubMed] [Google Scholar]
- 107.Qin W., Chen Q., Xi L. A handheld microscope integrating photoacoustic microscopy and optical coherence tomography. Biomed. Opt. Express. 2018;9(5):2205–2213. doi: 10.1364/BOE.9.002205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guo H., Chen Q., Qi W.Z., Chen X.X., Xi L. In vivo study of rat cortical hemodynamics using a stereotaxic‐apparatus‐compatible photoacoustic microscope. J. Biophotonics. 2018;11(9) doi: 10.1002/jbio.201800067. [DOI] [PubMed] [Google Scholar]
- 109.Qi W.Z., Chen Q., Guo H., Xie H.K., Xi L. Miniaturized optical resolution photoacoustic microscope based on a microelectromechanical systems scanning mirror. Micromachines. 2018;9(6):288. doi: 10.3390/mi9060288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen Q., Guo H., Qi W.Z., Gan Q., Yang L., Ke B.W., Chen X.X., Jin T., Xi L. Assessing hemorrhagic shock: feasibility of using an ultracompact photoacoustic microscope. J. Biophotonics. 2018;12(4) doi: 10.1002/jbio.201800348. [DOI] [PubMed] [Google Scholar]
- 111.Chen Q., Xie H.K., Xi L. Wearable optical resolution photoacoustic microscopy. J. Biophotonics. 2019;12(8) doi: 10.1002/jbio.201900066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lu C., Xiong K.D., Ma Y.Z., Zhang W.Y., Cheng Z.W., Yang S.H. Electrothermal-MEMS-induced nonlinear distortion correction in photoacoustic laparoscopy. Opt. Express. 2020;28(10):15300–15313. doi: 10.1364/OE.392493. [DOI] [PubMed] [Google Scholar]
- 113.Zhang W.Y., Ma H.G., Cheng Z.W., Wang Z.Y., Xiong K.D., Yang S.H. High-speed dual-view photoacoustic imaging pen. Opt. Lett. 2020;45(7):1599–1602. doi: 10.1364/OL.388863. [DOI] [PubMed] [Google Scholar]
- 114.Lin L., Zhang P.F., Xu S., Shi J.H., Li L., Yao J.J., Wang L.D., Zou J., Wang L.V. Handheld optical-resolution photoacoustic microscopy. J. Biomed. Opt. 2017;22(4) doi: 10.1117/1.JBO.22.4.041002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Park K., Kim J.Y., Lee C., Jeon S., Lim G., Kim C. Handheld photoacoustic microscopy probe. Sci. Rep. 2017;7:13359. doi: 10.1038/s41598-017-13224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qin W., Jin T., Guo H., Xi L. Large-field-of-view optical resolution photoacoustic microscopy. Opt. Express. 2018;26(4):4271–4278. doi: 10.1364/OE.26.004271. [DOI] [PubMed] [Google Scholar]
- 117.Kim J.Y., Lee C., Park K., Lim G., Kim C. Fast optical-resolution photoacoustic microscopy using a 2-axis water-proofing MEMS scanner. Sci. Rep. 2015;5:7932. doi: 10.1038/srep07932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Qi W.Z., Jin T., Rong J., Jiang H.B., Xi L. Inverted multiscale optical resolution photoacoustic microscopy. J. Biophotonics. 2017;10(12):1580–1585. doi: 10.1002/jbio.201600246. [DOI] [PubMed] [Google Scholar]
- 119.Yao J.J., Huang C.H., Wang L.D., Yang J.M., Gao L., Maslov K.I., Zhou J., Wang L.V. Wide-field fast-scanning photoacoustic microscopy based on a water-immersible MEMS scanning mirror. J. Biomed. Opt. 2012;17(8) doi: 10.1117/1.JBO.17.8.080505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huang C.H., Yao J.J., Wang L.V., Zhou J. A water-immersible 2-axis scanning mirror microsystem for ultrasound andha photoacoustic microscopic imaging applications. Microsystem Technol. 2013;19(4):577–582. doi: 10.1007/s00542-012-1660-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim J., Lee C., Park K., Lim G., Kin C. A PDMS-Based 2-Axis waterproof scanner for photoacoustic microscopy. Sensors. 2015;15(5):9815–9826. doi: 10.3390/s150509815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou L., Wang D.K., Xie H.K. An electrothermal micromirror with J-shaped bimorph microactuators. 2019 International Conference on Optical MEMS and Nanophotonics (OMN); IEEE; 2019. 266-227. [Google Scholar]
- 123.Xi L., Sun J.J., Zhu Y.P., Wu L., Xie H.K., Jiang H.B. Photoacoustic imaging based on MEMS mirror scanning. Biomed. Opt. Express. 2010;1(5):1278–1283. doi: 10.1364/BOE.1.001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xi L., Grobmyer S.R., Wu L., Chen R.M., Zhou G.Y., Gutwein L.G., Sun J.J., Liao W.J., Zhou Q.F., Xie H.K., Jiang H.B. Evaluation of breast tumor margins in vivo with intraoperative photoacoustic imaging. Opt. Express. 2012;20(8):8726–8731. doi: 10.1364/OE.20.008726. [DOI] [PubMed] [Google Scholar]
- 125.Yang H., Xi L., Samuelson S., Xie H.K., Yang L., Jiang H.B. Handheld miniature probe integrating diffuse optical tomography with photoacoustic imaging through a MEMS scanning mirror. Biomed. Opt. Express. 2013;4(3):427–432. doi: 10.1364/BOE.4.000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xi L., Duan C., Hk Xie, Jiang H.B. Miniature probe combining optical-resolution photoacoustic microscopy and optical coherence tomography for in vivo microcirculation study. Appl. Opt. 2013;52(9):1928–1931. doi: 10.1364/AO.52.001928. [DOI] [PubMed] [Google Scholar]
- 127.Guo H., Song C.L., Xie H.K., Xi L. Photoacoustic endomicroscopy based on a MEMS scanning mirror. Opt. Lett. 2017;42(22):4615–4618. doi: 10.1364/OL.42.004615. [DOI] [PubMed] [Google Scholar]
- 128.Yao J.J., Wang L.D., Yang J.M., Maslov K.I., Wong T.T., Li L., Huang C.H., Zou J., Wang L.V. High-speed label-free functional photoacoustic microscopy of mouse brain in action. Nat. Methods. 2015;12(5):407–410. doi: 10.1038/nmeth.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.He Y., Wang L.D., Shi J.H., Yao J.J., Li L., Zhang R.Y., Huang C.H., Zhou J., Wang L.V. In vivo label-free photoacoustic flow cytography and on-the-spot laser killing of single circulating melanoma cells. Sci. Rep. 2016;6 doi: 10.1038/srep39616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tan A.C.S., Tan G.S., Denniston A.K., Keane P.A., Ang M., Milea D., Chakravarthy U., Cheung C.M.G. An overview of the clinical applications of optical coherence tomography angiography. EYE. 2018;32(2):262–286. doi: 10.1038/eye.2017.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhi W.X., Wang Y., Chang C., Wang F., Chen Y.L., Hu N., Zhu X.L., Xie L. US-guided diffuse optical tomography: clinicopathological features affect total hemoglobin concentration in breast cancer. Trans. Oncol. 2018;11(4):845–851. doi: 10.1016/j.tranon.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Khashru M.B.B., Wang Z.T., Akthar B., Talukder M.F. A review of the clinical applications and performance of laser speckle contrast imaging. Int. J. Scientific Reports. 2020;6(2):77. doi: 10.18203/issn.2454-2156.IntJSciRep20200199. [DOI] [Google Scholar]
- 133.Zeng L.M., Liu G.D., Yang D.W., Ji X.R. Portable optical-resolution photoacoustic microscopy with a pulsed laser diode excitation. Appl. Phys. Lett. 2013;102(5) doi: 10.1063/1.4791566. [DOI] [Google Scholar]
- 134.Zhang X.Y., Koppal S.J., Zhang R., Zhou L., Butler E., Xie H.K. Wide-angle structured light with a scanning MEMS mirror in liquid. Opt. Express. 2016;24(4):3479–3487. doi: 10.1364/OE.24.003479. [DOI] [PubMed] [Google Scholar]
- 135.Lmai T., Shi J.H., Wong T.T.W., Li L., Zhu L.R., Wang L.V. High-throughput ultraviolet photoacoustic microscopy with multifocal excitation. J. Biomed. Opt. 2018;23(3) doi: 10.1117/1.JBO.23.3.036007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li Y., Wong T.T.W., Shi J.H., Hsu H.C., Wang L.V. Multifocal photoacoustic microscopy using a single-element ultrasonic transducer through an ergodic relay. Light Sci. Appl. 2020;9(1):135. doi: 10.1038/s41377-020-00372-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bell K.L., Hajireza P., Shi W., Zemp R.J. Temporal evolution of low-coherence reflectrometry signals in photoacoustic remote sensing microscopy. Appl. Opt. 2017;56(18):5172–5181. doi: 10.1364/AO.56.005172. [DOI] [PubMed] [Google Scholar]
- 138.Hajireza P., Shi W., Bell K., Paproski R.J., Zemp R.J. Non-interferometric photoacoustic remote sensing microscopy. Light Sci. Appl. 2017;6(6) doi: 10.1038/lsa.2016.278. e16278-e16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bell K., Hajireza P., Zemp R.J. Scattering cross-sectional modulation in photoacoustic remote sensing microscopy. Opt. Lett. 2018;43(1):146–149. doi: 10.1364/OL.43.000146. [DOI] [PubMed] [Google Scholar]