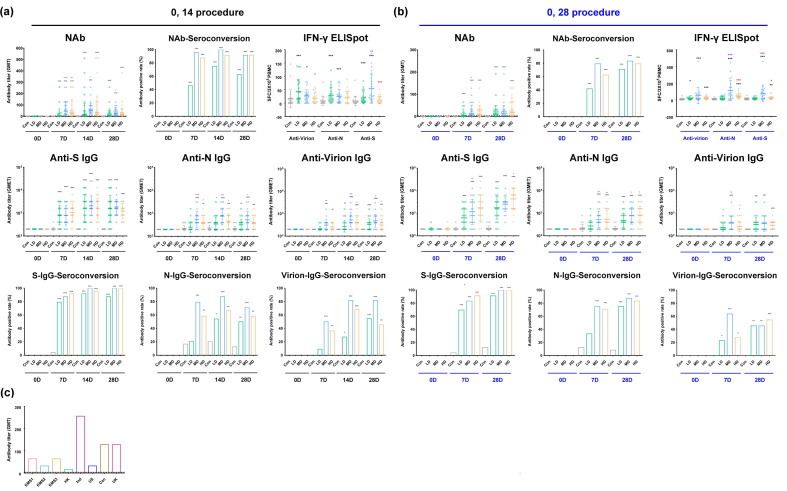
Fig. 4.
Immune responses induced in human individuals immunized with inactivated SARS-Cov-2 vaccine in the 0, 14 and 0, 28 schedule groups. (a) Neutralizing antibodies (NAb) and ELISA antibodies (IgGs) against the S protein (Anti-S IgG), N protein (Anti-N IgG) and virion (Anti-Virion IgG) and specific positive CTL responses (IFN-γ ELISpot) against the S, N and virion antigens (displayed on abscissa) induced by the inactivated vaccine in individuals assigned to the immunization procedure with an interval of 14 days after booster immunization. The corresponding seroconversion rate is also shown in the figure. (b) Neutralizing antibodies (NAb) and ELISA antibodies (IgGs) against the S protein (Anti-S IgG), the N protein (Anti-N IgG) and virion (Anti-Virion IgG) and specific positive CTL responses (IFN-γ ELISpot) against the S, N and virion antigens (displayed on abscissa) induced by the inactivated vaccine in individuals assigned to the immunization procedure with an interval of 28 days after booster immunization. The corresponding seroconversion rate is also shown in the figure. (c) Neutralizing antibodies induced by the inactivated vaccine could identify pandemic strains from all over the world, including the strain carrying the D614G mutation from America and strains from Hong Kong, Indonesia, Canada, and the United Kingdom with the same S protein sequence as that of the strain used to produce the vaccine. Control (Con, 0 EU); low dose (LD, 50 EU); medium dose (MD, 100 EU); and high dose (HD, 150 EU). The antibody positive judgment threshold is marked with a dotted line in the figure. *, 0.01 < p < 0.05; **, 0.001 < p < 0.01; ***, p < 0.001. The significant differences compared to the control group (Con) are shown in black, those compared to the low-dose group in purple, and those compared to the middle-dose group in red.