FIGURE 2.
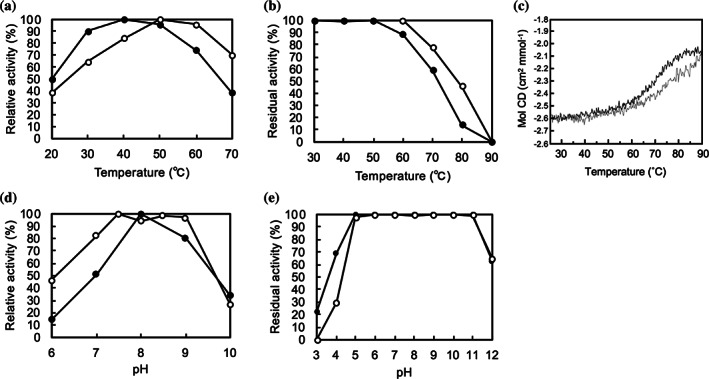
Comparison of enzyme properties of LGOX R305E with wild‐type LGOX. (a) Temperature dependence of the enzyme activities of LGOX R305E (closed circle) and wild‐type LGOX (open circle). Relative activities at pH 7.4 from 20°C to 70°C are plotted. The maximal activity is set as 100% relative activity (40°C for LGOX R305E and 50°C for wild‐type LGOX). (b) The remaining activities after incubation at various temperatures (30–70°C) for 1 h. Relative activities of LGOX R305E (closed circle) and wild‐type LGOX (open circle) are plotted. The activity without heat treatment was set as 100% remaining activity. (c) Far‐UV CD curves of LGOX R305E (black) and wild‐type LGOX (gray) at 210 nm obtained by temperature scan from 25°C to 90°C. (d) Effect of pH on the activity of LGOX R305E (closed circle) and wild‐type LGOX (open circle). The activity at optimum pH was set as 100% relative activity. (d) The remaining activities after incubation at various pH for 1 h on ice on stability. Relative activities of LGOX R305E (closed circle) and wild‐type LGOX (open circle) are plotted. The activity at pH 7.4 was set as 100% remaining activity
