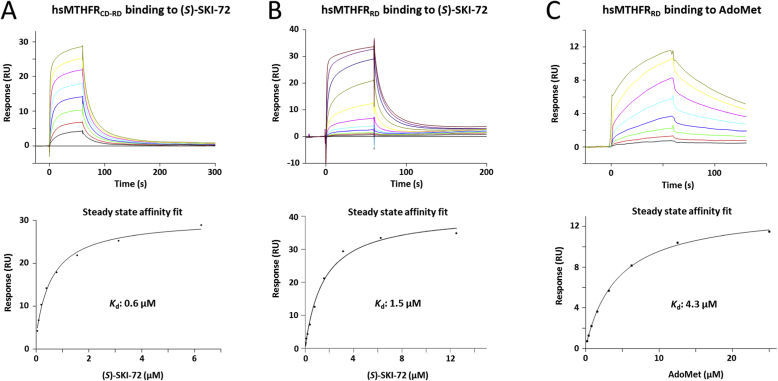
Fig. 3.
Characterization of hsMTHFRRD-CDand hsMTHFRRDbinding to (S)-SKI-72 and/or AdoMet via SPR. A-C. Above: Representative sensorgram plots of response units (RU) against time for different concentrations of the ligands. Below: sensorgram plots of response against ligand concentration. Data were fitted using steady state affinity equation. Binding affinity indicated by the dissociation constant (Kd). Each curve is a representative of n = 2 replicates. Complete data: hsMTHFRCD-RD binds (S)-SKI-72: Kd = 596 nM and 612 nM hsMTHFRRD binds (S)-SKI-72: Kd = 1.47 μM and 1.175 μM hsMTHFRRD binds AdoMet: Kd = 1.057 μM and 4.3 μM.