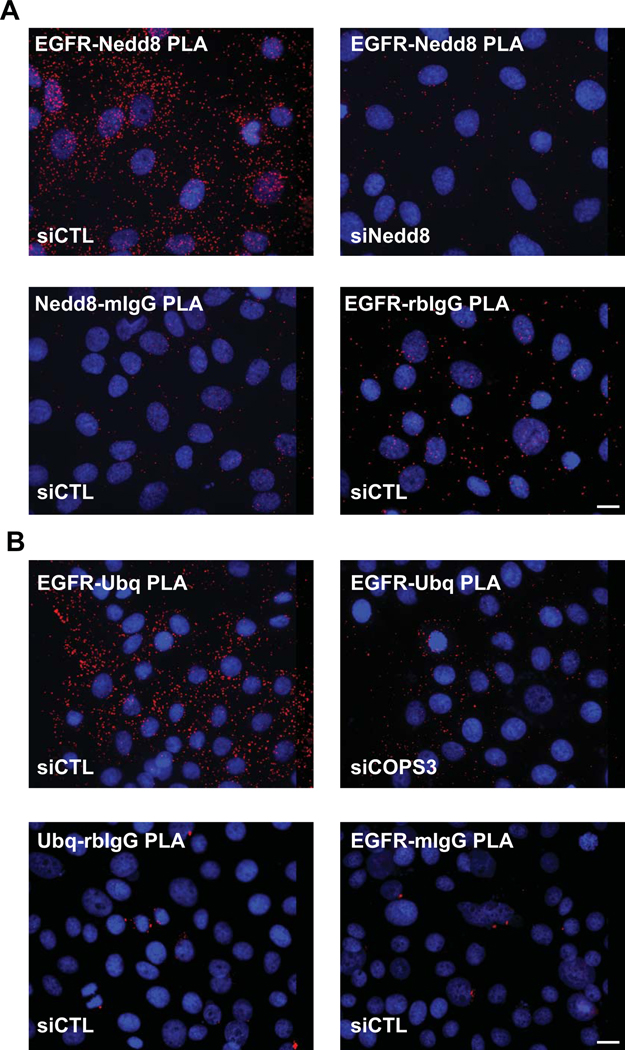
Figure 3. Utilizing PLA to detect specific post-translational protein modifications in situ.
EGFR is stabilized at the plasma membrane in part by the process of neddylation, the addition of Nedd 8 moieties on the C-terminus. De-neddylation is mediated by a large protein complex, the COPS9 signalosome, and results in the ubiquitination of EGFR and turnover of the receptor (Najor et al., 2017). A. PLA using an antibody pair recognizing EGFRmouse and the Nedd 8 moietyrabbit demonstrate the level of the post-translational modification of EGFR in the epithelial cells, visualized by PLA reaction “spots” shown red (Duolink™ In Situ Detection Reagent Red, λEx: 594, λEm: 624). Knock down of Nedd8 using siRNA technology resulted in the marked decrease in PLA signal. Other negative controls include the pairing of each specific antibody with a non-specific IgG counterpart (EGFRmouse:IgGrabbit or Nedd8rabbit:IgGmouse). DAPI labels nuclei in blue. B. De-neddylated EGFR (in siCONT panel) results in the ubiquitination of the receptor as seen by the strong PLA signal shown in red (Duolink™ In Situ Detection Reagent Red, λEx: 594, λEm: 624) when anti-EGFRrabbit is paired with anti-Ubiquitinmouse. As this process is regulated by the COPS9 signalosome, loss of an obligate constituent of the complex (siCOPS3 panel) results in a decrease in the EGFR ubiquitination, demonstrated by a marked decrease in PLA signal. Other controls shown: the pairing of each specific antibody with a non-specific IgG counterpart (EGFRrabbit:IgGmouse or Ubqmouse:IgGrabbit). DAPI labels nuclei in blue. Images were acquired using a DMR Leica microscope with a 40X (PL Fluotar, NA 1.0) using a Hamamatsu Orca 100 CCD camera, model C4742–95. Scale bar = 10 μm.