Abstract
Bacterial biofilms are a major threat to human health, causing persistent infections that lead to millions of fatalities worldwide every year. Biofilms also cause billions of dollars of damage annually by interfering with industrial processes. Recently, cationic pillararenes were found to be potent inhibitors of biofilm formation in Gram-positive bacteria. To identify the structural features of pillararenes that result in antibiofilm activity, we evaluated the activity of 16 cationic pillar[5]arene derivatives including that of the first cationic water-soluble pillar[5]arene-based rotaxane. Twelve of the derivatives were potent inhibitors of biofilm formation by Gram-positive pathogens. Structure activity analyses of our pillararene derivatives indicated that positively charged head groups are critical for the observed antibiofilm activity. Although certain changes in the lipophilicity of the substituents on the positively charged head groups are tolerated, dramatic elevation in the hydrophobicity of the substituents or an increase in steric bulk on these positive charges abolishes the antibiofilm activity. An increase in the overall positive charge from 10 to 20 did not affect the activity significantly, but pillararenes with 5 positive charges and 5 long alkyl chains had reduced activity. Surprisingly, the cavity of the pillar[n]arene is not essential for the observed activity, although the macrocyclic structure of the pillar[n]arene core, which facilitates the clustering of the positive charges, appears important. Interestingly, the compounds found to be efficient inhibitors of biofilm formation were nonhemolytic at concentrations that are ∼100-fold of their MBIC50 (the minimal concentration of a compound at which at least 50% inhibition of biofilm formation was observed compared to untreated cells). The structure–activity relationship guidelines established here pave the way for a rational design of potent cationic pillar[n]arene-based antibiofilm agents.
Keywords: bacterial biofilm, antibiofilm agents, cationic pillararenes, SAR, Gram-positive
Persistent and chronic infections are serious universal threats to humans, taking millions of lives each year. The National Institutes of Health (NIH) revealed that approximately 80% of recurrent microbial infections in the human body are associated with bacterial biofilms.1−4 Bacterial biofilms are microbial colonies, which are embedded in a self-produced matrix of extracellular polymeric substances (EPSs) that protect the microorganism from harsh environmental conditions, antibiotics, and the host immune system.5−8 Bacterial biofilms are very common modes of life that can form in most environments on earth and, therefore, may affect many aspects of modern healthcare and industrial processes.1−8 In the human body, for example, dental plaque is one of the most well-known and prevalent examples of a bacterial biofilm. These biofilms form on tooth surfaces, and bacterial metabolism in plaque causes tooth decay and gum disease.9 Microbial cells within biofilms are more resistant to antibiotics than planktonic cells.10−12 Biofilm-associated infections can occur on damaged tissues such as wounds and burns and during lung, cardiac valve, or urinary tract infections. In addition, bacterial biofilm can develop on biomedical implants and devices such as sutures, heart valves, catheters, contact lenses, and dental implants.13−16
In recent years, there has been a constant search for new antibiofilm agents, which will effectively inhibit biofilm formation for a long period. This can be achieved by developing antivirulent agents that inhibit biofilm formation, thus reducing the risk for the development of bacterial resistance to these agents. As reported previously, cationic amphiphiles,17−21 especially quaternary ammonium cations (QACs), are known to be one of the most potent families of antibacterial and antibiofilm agents.19,20 In recent years, there have been many attempts to develop cationic amphiphilic agents with improved antibiofilm activity17−22 and reduced toxicity to mammalian cells.22−26 For example, Böttcher et al. reported the synthesis of cationic amphiphilic compounds bearing guanidinium and bis-guanidinium groups, which prevent biofilm formation of Bacillus subtilis and Staphylococcus aureus strains.22 Jennings et al. synthesized a library of QACs that serve as simple antimicrobial peptides mimics.23,24 These compounds had antimicrobial activity against several Gram-positive and Gram-negative bacterial strains. Some of these compounds efficiently eradicate existing biofilms of S. aureus and Enterococcus faecalis, but many of them were found to be highly hemolytic.23 More recently, antimicrobial QACs that are much less hemolytic were reported.26 Haldar and co-workers prepared cationic amphiphiles composed of chains of variable lengths bearing bis-ammonium cations that inhibited and eradicated biofilms of S. aureus and Escherichia coli strains without bactericidal or acute mammalian cell toxicity.27,28 Some of the compounds were even tested under in vivo conditions.28−30 It should be noted, however, that these compounds are also potent antimicrobials; thus, their antibiofilm activity likely results from the eradication of bacteria rather than direct intervention in the biofilm formation processes. As discussed by Melander and co-workers, there is a need for antibiofilm agents that have no effect on bacterial cell growth as such agents should not result in drug resistance.31 Lately, several examples of emergence of bacterial resistance against QACs were reported.25,26
The pillar[n]arene family was introduced more than a decade ago, and since then, there has been a rising interest in these new macrocycles.32,33 Pillar[n]arenes possess a unique set of properties, such as a symmetric tubular structure that can be easily functionalized at both rims with various functional groups.32,33 Their ease of synthesis and functionalization increase their popularity and have made them a widely used family of macrocycles with applications spanning different fields from biology to material sciences.34−42 Pillar[n]arenes are used as drug delivery systems,35 as separating agents,37,38 as light harvesting systems,36,41 as ions channels mimics,36,42 and as a scaffold for new materials such as supramolecular gels34 and polymers.39 In recent years, we showed that pillararene derivatives can be used to complex xenon in water43 and to form rim-to-rim supramolecular organogels44 as well as hydrogen bond-based supramolecular boxes in water.45
Recently, we found that cationic pillar[n]arenes (n = 5, 6) are potent inhibitors of biofilm formation of clinically important Gram-positive pathogens (Figure 1).46,47 Interestingly, although bearing several QACs, these cationic pillar[n]arenes show no effect on bacterial cell viability and cause no damage to red blood cells (RBCs) and no acute toxicity to human cells in culture at concentrations that are orders of magnitude higher than their antibiofilm active concentrations.46,47
Figure 1.

Biofilm formation and inhibition and the structural features tested during evaluation of the structure activity relationship of pillararene derivatives as inhibitors of biofilm formation.
Our previous studies suggested that the positive charges are the key for the observed antibiofilm activity.46,47 Very recently, Gao et al. demonstrated that a zwitterionic pillar[5]arene derivative has antimicrobial activity against the Gram-positive S. aureus (SH1000) and the Gram-negative E. coli (DH5a) strain.48 The zwitterionic pillar[5]arene derivative eradicated the pre-existing biofilm formed by an E. coli strain, albeit at high concentrations.48 In the present study, with the goal of establishing rules for the design of cationic pillararenes capable of inhibiting biofilm formation, we prepared and evaluated the antibiofilm activity of 16 cationic pillararene derivatives (15 new compounds and one reference). Therefore, it allowed us to establish a comprehensive structure activity relationship (SAR) of the pillararene derivatives that inhibit biofilm formation by clinically important Gram-positive pathogens.
Results
Synthesis of Cationic Pillararene Derivatives
To explore the effect of different structural characteristics of the cationic pillararenes on their antibiofilm activity, we designed and synthesized the cationic pillar[5,6]arene derivatives presented in Scheme 1. Compounds 1–14 were synthesized according to previously described procedures with some modifications.46,47 For synthetic procedures and characterization data, see Schemes S1–S3 and Figures S1–S51. Compound 15, which is a water-soluble cationic pillar[5]arene-based rotaxane obtained from the previously synthesized cationic pillar[5]arene 1, was prepared as shown in Scheme S4. Briefly, 15 was assembled by initially threading a dodecyl chain bearing one DABCO (1,4-diazabicyclo[2.2.2]octane) group and a bromide group (15a) into the cavity of pillar[5]arene 1 in water affording the pseudo rotaxane, which was then reacted with a second DABCO group to form the mechanically locked rotaxane 15. The synthesis and the characterization of rotaxane 15 are presented in Scheme S4 and Figures S52–S55. To the best of our knowledge, this is the first example for the preparation of a polycationic pillar[n]arene-based rotaxane.49
Scheme 1. Cationic Pillar[5,6]arene Derivatives 1–15 Discussed in This Work.

Inhibition of Biofilm Formation
To evaluate the ability of our cationic pillararenes to inhibit the formation of biofilm, we focused on two clinically important biofilm forming Gram-positive pathogens: methicillin-resistant S. aureus (ATCC 33592) and E. faecalis (ATCC 29212).50−53 We evaluated the entire dose response for all our compounds using the crystal violet protocol46,47,54 from which the MBIC50, i.e., the minimal concentration of a compound at which at least 50% inhibition of biofilm formation was observed compared to untreated cells, was computed. Images of the raw data are shown in Figures S56–S63. The quantitative analysis of the biofilm inhibition activity is presented in Figures 2 and S64–S67 ,and the extracted MBIC50 values are summarized in Table 1. Note that compounds 2–15 were evaluated for the first time, and compound 1(46) was tested as a reference. With the exception of pillararenes 4, 7, 14a, and 14b, the cationic pillar[5,6]arene derivatives effectively inhibited the formation of biofilms by both S. aureus and E. faecalis. These results indicate that increasing the chain length of one or two of the substituents on the ammonium groups to butyl groups had a limited effect on the activity. However, when the hydrophobicity and size of three substituents of the cationic head groups were increased considerably (i.e., compounds 4 or 7), the activity was fully abrogated. Compound 8, with a longer spacer between the pillararene scaffold and the cationic head group, had an activity identical to 1. Interestingly, alternation of the type of cationic head group (compare compounds 1, 10, 11, and 12) or an increase in the number of positively charged head groups (compare compounds 1 and 12 with compound 13) had little effect on the inhibition of biofilm formation. Rotaxane 15 had similar to or slightly higher activity than 1; both had MBIC50 values in the sub-micromolar range. Compound 14a, which is a mixture of isomers, and compound 14b, which is only the symmetric isomer, that have 5 positive charges, were about an order of magnitude less potent than the other compounds, which each have 10 or more positive charges.
Figure 2.

Biofilm formation by (a) S. aureus ATCC 33592 (MRSA) and (b) E. faecalis ATCC 29212 evaluated using the double dilution method (final OD600 = 0.1) in the presence of different concentrations of compounds 1–4 and 6. Values are mean ± standard error of at least 3 independent experiments of 5 repetitions each.
Table 1. Biofilm Inhibitory Activity of Cationic Pillar[5,6]arene Derivatives and Predicted Octanol–Water Distribution Coefficientsa.
| MBIC50 values in μM (μg/mL) |
|||
|---|---|---|---|
| compound | S. aureus ATCC 33592 | E. faecalis ATCC 29212 | LogD |
| 1 | 0.45 (1) | 0.45 (1) | –32.55 |
| 2 | 0.37 (1) | 0.19 (0.5) | –19.32 |
| 3 | 0.31 (1) | 0.31 (1) | –6.08 |
| 4 | >8.7 (>32) | >8.7 (>32) | 7.16 |
| 5 | 0.61 (2) | 0.61 (2) | –17.09 |
| 6 | 0.32 (1) | 0.32 (1) | –10.42 |
| 7 | >7.72 (>32) | >7.72 (>32) | 16.25 |
| 8 | 0.35 (1) | 0.35 (1) | –17.89 |
| 9 | 0.69 (2) | 0.69 (2) | –38.35 |
| 10 | 0.63 (2) | 0.63 (2) | –32.32 |
| 11 | 0.40 (1) | 0.40 (1) | –24.71 |
| 12 | 0.71 (2) | 0.36 (1) | –35.89 |
| 13 | 0.95 (4) | 0.47 (2) | –77.42 |
| 14a | >14.94 (>32) | >14.94 (>32) | 8.12 |
| 14b | 14.94 (32) | 7.47 (16) | 8.12 |
| 15 | 0.35 (1) | 0.18 (0.5) | –37.20 |
Each MBIC50 value is a mean of at least three independent experiments, each including five replicates of each concentration.
Hemolytic Activity
The cationic amphiphilic nature of the pillararenes in this study suggests that these compounds could be potentially hemolytic. Therefore, the hemolytic effects of compounds 1–8 and 10–15 were evaluated in assays with rat RBCs. The positive controls were Triton X-100 and cetrimonium bromide (CTAB). The results are presented in Figure S68. The concentrations that caused 50% hemolysis of the RBCs (HC50 values) are summarized in Figure 3. Interestingly, all the active compounds tested, i.e., compounds 1–3, 5, 6, 8, 11–13, and 15, did not lyse red blood cells even at 256 μg/mL, the highest concentration tested, which is more than 100-fold higher than the MIBC50 values of these compounds. Compound 10 showed about 6% hemolysis at a concentration of 256 μg/mL. Notably, compounds 4, 7, 14a, and 14b were hemolytic with HC50 values similar to that of CTAB.
Figure 3.
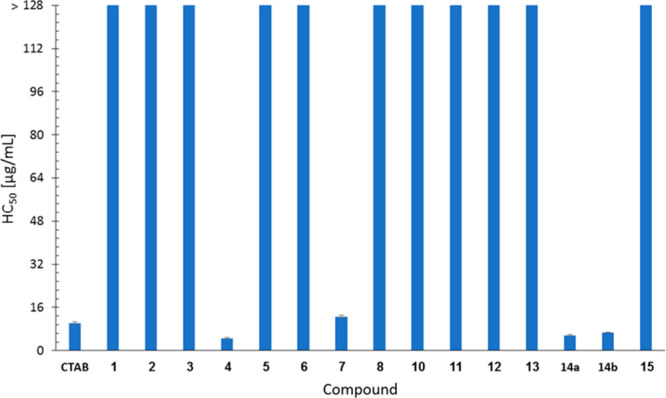
Hemolytic activities of cationic pillar[n]arenes 1–8 and 10–15. Test compounds were added to rat RBCs suspended in PBS buffer; a range of concentrations was evaluated. After 1 h at 37 °C, the percentage of hemoglobin released relative to cells treated with Triton X100 (100% hemolysis) was quantified by measuring the absorbance at 550 nm. Each concentration was tested in triplicate, and the results are expressed as means ± standard error from two independent experiments.
Effect on Bacterial Growth
To confirm that the new derivatives do not affect bacterial growth, bacterial growth curve analysis was performed for 3 of the most potent compounds, i.e., compounds 2, 12, and 15. The results are presented in Figures S70–S73. As expected and in line with previous observations,46,47 the compounds did not affect the bacterial growth even at the highest tested concentration, 64 μg/mL, which is significantly higher than the MBIC50.
Discussion
The analysis of the antibiofilm activity of compounds 1–15 as well as compounds 16–21 reported previously (Table S1)46,47 revealed the structural features of cationic pillar[n]arenes that are important for the inhibition of biofilm formation by Gram-positive bacteria.
An increase in the hydrophobicity of the cationic pillar[n]arenes was accomplished by varying the aliphatic substituents on the ammonium head groups. Inhibition of biofilm formation by pillar[n]arenes 1, 2, 3, 5, 6, and 16 was similar, whereas compounds 4 and 7 were inactive. These results indicate that an increase in the hydrophobicity had a small effect on the antibiofilm activity. The lack of activity of 4 was likely due to the inaccessibility of the positively charged head groups of the pillar[n]arenes as a result of the steric bulk caused by the aliphatic chains attached to the cationic center. Similarly, in cationic pillararene 7, the C12-alkyl chains reduce the accessibility to the positively charged head groups. The predicted distribution coefficient (LogD) is a measure of hydrophobicity. Comparisons of the LogD values revealed that the four pillar[n]arenes that displayed poor inhibition of biofilm formation (4, 7, 14a, and 14b) were also the most hydrophobic of the cationic pillar[n]arenes synthesized. The LogD values for 4, 7, 14a, and 14b are between 7.16 and 16.25 (Table 1); the rest of the cationic pillar[n]arenes were orders magnitude more hydrophilic (LogD value range of −6.08 to −77.42).
We also explored the importance of the length of the linker between the pillar[n]arene scaffold and the ammonium group. The similarities of inhibitory activities of cationic pillar[5]arenes 1, 9, 18, and 8 suggest that changing the length of the spacer between the positively charged head groups and the pillararene scaffold from ethyl to propyl and to hexyl chain, respectively, did not significantly affect the inhibition of biofilm formation.
The chemical identity of the cationic head groups did not significantly affect the antibiofilm activity of the cationic pillar[n]arenes. For example, cationic pillar[5]arenes bearing ammonium and phosphonium and N-methyl imidazolium-, pyridinium- and DABCO-based cationic head groups (1, 20-21, 10, 11 and 12-13, respectively) had MBIC50 values in the range of ∼0.4–1.55 μM.
Another feature addressed is the importance of the number of positive charges in determining the antibiofilm activity. An increase in the number of positive charges by 20% from 10 to 12 had a negligible effect on the antibiofilm activity (compound 17).46 To test if a more dramatic increase in the number of positive charges affects antibiofilm activity, we generated a derivative with 20 positively charged head groups. N-Methylation of the DABCO head groups of cationic pillar[n]arene 12 with 10 positive charges gave derivative 13 with 20 positive charges. Interestingly, these two cationic pillar[n]arenes had a similar antibiofilm activity. These results suggest that, beyond a certain number of charges, the clustering of the charges on the pillararene scaffold rather than the number of charges per se, affects the antibiofilm activity. Earlier results indicated that the positive head groups should be clustered together and the monomeric units of the respective pillararenes, even at 5 times the concentrations used, were completely inactive.47
To challenge if reducing the number of positive head groups and placing them either on one or on both sides of the pillararene scaffold affects the antibiofilm activity, we tested the antibiofilm activity of 14a, the statistical mixture of all pillar[5]arene isomers having five positive head groups, and compared it to the activity of 14b, the symmetric isomer. Table 1 shows that both 14a and 14b were much less active. The fact that mixture 14a was even less active than 14b suggests that one cannot rule out that some of the decrease in the observed activity occurred because these systems have only 5 positive head groups. Importantly, one should also note that the LogD of 14a,b is in fact very different from all compounds found to be potent inhibitors of biofilm formation. Thus, it may well be that the reduction in the potency of these materials arises from their high lipophilicity.
Finally, we explored the importance of one of the unique features of the pillar[n]arene scaffold, which is the cavity. We therefore synthesized the water-soluble cationic pillar[5]arene-based rotaxane 15. Pillar[n]arenes can serve as good building blocks for rotaxanes and pseudorotaxanes due to their symmetrical rigid structure, π-rich cavity, and host–guest properties.17 In constructing rotaxane 15, we used cationic pillar[5]arene 1 as the wheel and a dodecyl chain with two DABCO groups on both ends as the dumbbell. The blockage of the cavity of the pillar[5]arene did not reduce the ability of the pillar[n]arene to inhibit the formation of the biofilms. MBIC50 values of rotaxane 15 were of the same order of magnitude as those of pillar[5]arene 1 from which it was generated, although the values of the former were slightly lower.
The evaluation of the antibiofilm activity of the collection of cationic pillar[n]arenes 1–21 revealed several structure–activity relationship features important for potent inhibition of biofilm formation by the cationic pillar[n]arenes: (1) accessibility of the positive charges (significant shielding of the positive charges reduced the activity of the cationic pillararenes); (2) the pillar[n]arene structure (the cavity of the pillar[n]arene is not essential for the inhibition of biofilm formation; however, clustering of the positive charges, which is dictated by the pillar[n]arene structure, is key for antibiofilm activity); (3) lipophilicity (some enhancement of lipophilicity due to the alteration of the substituents on the positively charged head groups is tolerated); (4) spacer (the distance between the pillar[n]arene macrocycle and the positive head groups can be modified through alteration of the aliphatic chain spacer); (5) cationic head group (the type of the positive head group does not affect the inhibition of biofilm formation); (6) net positive charge (variation from 10 to 20 positive head groups does not affect the activity of the cationic pillar[n]arenes; however, a reduction of this number to 5 positive charges while placing the lipophilic group in other positions decreases the activity by an order of magnitude); (7) LogD values (compounds having positive LogD values were found to be inactive).
Conclusion
In this study, we identified the structural determinants that affect the efficacy of cationic pillar[n]arenes in inhibiting biofilm formation by two important Gram-positive pathogens. Many of the tested compounds potently inhibited biofilm formation, and some were completely inactive. Importantly, we found that a plurality of accessible positive charges and not their nature are important determinants for the observed antibiofilm activity of these compounds. We showed that the multiplication of the number of positive charges from 10 to 20 did not increase the activity; however, a reduction in the number of positive head groups combined with an increase in the lipophilicity of the compound decreased the antibiofilm activity of the cationic pillararenes. Importantly, we provided evidence that the cavity of the pillararene that can serve as a host for small molecules and aliphatic chains is not essential for the inhibition of biofilm formation. However, the clustering of the positive charges on the pillararene skeleton is important. Interestingly, the compounds that are potent inhibitors of biofilm formation were also found to be nonhemolytic and to have no effect on bacterial cell growth. Therefore, it will be interesting to study if these compounds keep their biofilm inhibition activity for a longer period compared to other agents. In addition, it will be important to test if any synergistic effect can be observed when such cationic pillararene agents are administered along with known antibiotics in drug-resistant bacterial strains. These findings and the conclusions drawn thereof will guide the design of more potent and active pillararene-based materials for the inhibition of biofilms by Gram positive bacteria.
Methods
Materials
Starting materials were purchased from Sigma-Aldrich, Alfa Aesar, TCI, Cambridge Isotope Laboratories, and Bio-Lab Ltd. and used as received. Chemical reactions were monitored by TLC (Merck, silica gel 60 F254), and the compounds were purified by SiO2 flash chromatography (Merck Kieselgel 60). 1H and 13C NMR spectra were recorded on 400 and 500 MHz Bruker Avance NMR spectrometers. All compounds were prepared according to Schemes S1–S4 using a modified procedure,46,47 and their full NMR and HRMS characterization appear in Schemes S1–S4 and Figures S1–S55. The purity of the compounds was determined by chemical analysis or HPLC and was higher than 95% (see the Supporting Information).
Biological Assays
Analysis of Biofilm Inhibition
The antibiofilm activity assay was performed as described previously46,47,54 with minor modifications. Briefly, the tested bacterial strains were grown from frozen stocks in brain heart infusion (BHI) medium overnight at 37 °C in 5% CO2. Then, 100 μL of serial 1:2 dilutions of each compound in Tryptic soy broth (TSB) + 1% glucose (32, 16, 8, 4, 2, 1, and 0.5 μg/mL) were prepared in flat-bottomed 96-well microplates (Costar, Corning). Control wells with no compounds and wells without bacteria containing each tested concentration of the compounds (blanks) were also prepared. An equal volume (100 μL) of bacterial suspensions in TSB + 1% glucose was added to each well to a final OD600 of 0.1. After incubation for 24 h at 37 °C in 5% CO2 under aerobic conditions, spent media and free-floating bacteria were removed by turning over the plates. The wells were vigorously rinsed at least four times with doubly distilled water (DDW).
Crystal Violet Assay
0.4% Crystal violet (200 μL) solution was added to each well. After 45 min, wells were vigorously rinsed three times with DDW to remove unbound dye. After adding 200 μL of 33% acetic acid to each well, the plate was shaken for 15 min to release the dye. Biofilm formation was quantified by measuring the difference between the absorbance of untreated and treated bacterial samples for each tested concentration of the compounds and the absorbance of the appropriate blank well at 600 nm (A600) using a Tecan plate reader. The MBIC50 was defined as the lowest concentration at which at least 50% reduction in biofilm formation was measured compared to untreated cells. Each concentration of compound was tested in five replicates, and at least three independent experiments were performed.
Rat Red Blood Cell Hemolysis Assay
The hemolysis was performed as previously described with minor modifications.55 Briefly, a sample of rat red blood cells (2% w/w in PBS) was incubated with each of the tested compounds (CTAB and compounds 1–8 and 10–15) for 1 h at 37 °C in 5% CO2 using the double dilution method starting at a concentration of 256 μg/mL. The negative control was PBS, and the positive control was a 1% v/v solution of Triton X-100 (which induced 100% hemolysis). Following centrifugation (2000 rpm, 10 min, ambient temperature), the supernatant was removed and absorbance at 550 nm was measured using a microplate reader (Genios, TECAN). The graphs of the percentage of hemoglobin released vs the compounds’ concentrations, relative to the positive control (Triton X-100), were obtained from two independent experiments performed in triplicate.
Bacterial Growth Curve Analysis
The tested bacterial strains were first grown from the frozen stock in BHI broth for 24 h at 37 °C. Volumes of 100 μL of serial 1:2 dilutions (64, 32, 16, 8, 4, 2, and 1 μg/mL) of the selected compounds in TSB + 1% glucose were prepared in flat-bottomed 96-well microplates (Corning). Next, an equal volume (100 μL) of bacterial suspension in TSB + 1% glucose was added to each well to a final OD600 of 0.01. Control wells with no compounds and wells without bacteria (blanks) were also prepared. During a 24 h incubation at 37 °C, growth kinetics were monitored by recording the optical density at a wavelength of 600 nm (OD600) using a Tecan plate reader. Each concentration was tested in triplicate, and the results are shown as an average of two independent experiments.
Acknowledgments
Y.C. thanks the Israel Science Foundation (ISF, Jerusalem, Israel, (grant no. 1006/19)) for partial financial support.
Glossary
Abbreviations
- CTAB
cetrimonium bromide
- DABCO
1,4-diazabicyclo-[2.2.2]octane
- HC50
concentration that results in 50% lysis of RBCs
- MBIC50
the minimal concentration of a compound at which at least 50% inhibition of biofilm formation was observed compared to untreated cells
- NMR
nuclear magnetic resonance
- RBCs
red blood cells
- HPLC
high pressure liquid chromatography
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsinfecdis.0c00662.
Synthetic procedures and spectroscopic characterization, including 1H and 13C NMR spectra of all compounds used in the study as well as descriptions of the biological tests and the results obtained for compounds 1–15 (PDF)
Author Present Address
∇ R.J.: Department of Chemistry, University of Calicut, Calicut, Kerala 673635, India.
Author Contributions
○ D.K.-K. and M.H. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Costerton J. W.; Stewart P. S.; Greenberg E. P. (1999) Bacterial biofilms: a common cause of persistent infections. Science 284, 1318–1322. 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- Donlan R. M. (2002) Biofilms: microbial life on surfaces. Emerging Infect. Dis. 8, 881–890. 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin N.; Zheng Y.; Opoku-Temeng C.; Du Y.; Bonsu E.; Sintim H. O. (2015) Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 7, 493–512. 10.4155/fmc.15.6. [DOI] [PubMed] [Google Scholar]
- Mah T.-F.; Pitts B.; Pellock B.; Walker G. C.; Stewart P. S.; O'Toole G. A. (2003) A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426, 306–310. 10.1038/nature02122. [DOI] [PubMed] [Google Scholar]
- Flemming H. C.; Wingender J.; Szewzyk U.; Steinberg P.; Rice S. A.; Kjelleberg S. (2016) Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 14, 563–575. 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- Flemming H. C.; Wingender J. (2010) The biofilm matrix. Nat. Rev. Microbiol. 8, 623–633. 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- Stoodley P.; Sauer K.; Davies D. G.; Costerton J. W. (2002) Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56, 187–209. 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- Zhang R.; Liu Y.; He M.; Su Y.; Zhao X.; Elimelech M.; Jiang Z. (2016) Antifouling membranes for sustainable water purification: strategies and mechanisms. Chem. Soc. Rev. 45, 5888–5924. 10.1039/C5CS00579E. [DOI] [PubMed] [Google Scholar]
- Steinberg D. (2016) Dental chatter: Bacterial cross-talk in the biofilm of the oral cavity. Isr. J. Chem. 56, 273–281. 10.1002/ijch.201400143. [DOI] [Google Scholar]
- Stewart P. S; William Costerton J (2001) Antibiotic resistance of bacteria in biofilms. Lancet 358, 135–138. 10.1016/S0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- Davies D. (2003) Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discovery 2, 114–122. 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- Koo H.; Allan R. N.; Howlin R. P.; Stoodley P.; Hall-Stoodley L. (2017) Targeting microbial biofilms: current and prospective therapeutic strategies. Nat. Rev. Microbiol. 15, 740–755. 10.1038/nrmicro.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L.; Costerton J. W.; Stoodley P. (2004) Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2, 95–108. 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- Lebeaux D.; Ghigo J.-M.; Beloin C. (2014) Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 78, 510–543. 10.1128/MMBR.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal M.; Ahmad W.; Andleeb S.; Jalil F.; Imran M.; Nawaz M. A.; Hussain T.; Ali M.; Rafiq M.; Kamil M. A. (2018) Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 81, 7–11. 10.1016/j.jcma.2017.07.012. [DOI] [PubMed] [Google Scholar]
- Bowen W. H.; Burne R. A.; Wu H.; Koo H. (2018) Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 26, 229–242. 10.1016/j.tim.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. (2002) Antimicrobial peptides of multicellular organisms. Nature 415, 389–395. 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- Zhang P.; Li S.; Chen H.; Wang X.; Liu L.; Lv F.; Wang S. (2017) Biofilm inhibition and elimination regulated by cationic conjugated polymers. ACS Appl. Mater. Interfaces 9, 16933–16938. 10.1021/acsami.7b05227. [DOI] [PubMed] [Google Scholar]
- Tischer M.; Pradel G.; Ohlsen K.; Holzgrabe U. (2012) Quaternary ammonium salts and their antimicrobial potential: targets or nonspecific interactions?. ChemMedChem 7, 22–31. 10.1002/cmdc.201100404. [DOI] [PubMed] [Google Scholar]
- Minbiole K. P. C.; Jennings M. C.; Ator L. E.; Black J. W.; Grenier M. C.; LaDow J. E.; Caran K. L.; Seifert K.; Wuest W. M. (2016) From antimicrobial activity to mechanism of resistance: the multifaceted role of simple quaternary ammonium compounds in bacterial eradication. Tetrahedron 72, 3559–3566. 10.1016/j.tet.2016.01.014. [DOI] [Google Scholar]
- Abouelhassan Y.; Basak A.; Yousaf H.; Huigens R. W. (2017) Identification of N-Arylated NH125 analogues as rapid eradicating agents against MRSA persister cells and potent biofilm killers of Gram-positive pathogens. ChemBioChem 18, 352–357. 10.1002/cbic.201600622. [DOI] [PubMed] [Google Scholar]
- Böttcher T.; Kolodkin-Gal I.; Kolter R.; Losick R.; Clardy J. (2013) Synthesis and activity of biomimetic biofilm disruptors. J. Am. Chem. Soc. 135, 2927–2930. 10.1021/ja3120955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings M. C.; Ator L. E.; Paniak T. J.; Minbiole K. P. C.; Wuest W. M. (2014) Biofilm-eradicating properties of quaternary ammonium amphiphiles: simple mimics of antimicrobial peptides. ChemBioChem 15, 2211–2215. 10.1002/cbic.201402254. [DOI] [PubMed] [Google Scholar]
- Jennings M. C.; Buttaro B. A.; Minbiole K. P. C.; Wuest W. M. (2015) Bioorganic investigation of multicationic antimicrobials to combat QAC-resistant Staphylococcus aureus. ACS Infect. Dis. 1, 304–309. 10.1021/acsinfecdis.5b00032. [DOI] [PubMed] [Google Scholar]
- Jennings M. C.; Minbiole K. P. C.; Wuest W. M. (2015) Quaternary ammonium compounds: an antimicrobial mainstay and platform for innovation to address bacterial resistance. ACS Infect. Dis. 1, 288–303. 10.1021/acsinfecdis.5b00047. [DOI] [PubMed] [Google Scholar]
- Morrison K. R.; Allen R. A.; Minbiole K. P. C.; Wuest W. M. (2019) More QACs, more questions: recent advances in structure activity relationships and hurdles in understanding resistance mechanism. Tetrahedron Lett. 60, 150935. 10.1016/j.tetlet.2019.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque J.; Konai M. M.; Samaddar S.; Gonuguntala S.; Manjunath G. B.; Ghosh C.; Haldar J. (2015) Selective and broad spectrum amphiphilic small molecules to combat bacterial resistance and eradicate biofilms. Chem. Commun. 51, 13670–13673. 10.1039/C5CC05159B. [DOI] [PubMed] [Google Scholar]
- Hoque J.; Konai M. M.; Sequeira S. S.; Samaddar S.; Haldar J. (2016) Antibacterial and antibiofilm activity of cationic small molecules with spatial positioning of hydrophobicity: an in vitro and in vivo evaluation. J. Med. Chem. 59, 10750–10762. 10.1021/acs.jmedchem.6b01435. [DOI] [PubMed] [Google Scholar]
- Hoque J.; Ghosh S.; Paramanandham K.; Haldar J. (2019) Charge-switchable polymeric coating kills bacteria and prevents biofilm formation in vivo. ACS Appl. Mater. Interfaces 11, 39150–39162. 10.1021/acsami.9b11453. [DOI] [PubMed] [Google Scholar]
- Konai M. M.; Haldar J. (2017) Fatty acid comprising Lysine conjugates: anti-MRSA agents that display in vivo efficacy by disrupting biofilms with no resistance development. Bioconjugate Chem. 28, 1194–1204. 10.1021/acs.bioconjchem.7b00055. [DOI] [PubMed] [Google Scholar]
- Worthington R. J.; Richards J. J.; Melander C. (2012) Small molecule control of bacterial biofilms. Org. Biomol. Chem. 10, 7457–7474. 10.1039/c2ob25835h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoshi T.; Kanai S.; Fujinami S.; Yamagishi T. A.; Nakamoto Y. (2008) para-Bridged symmetrical pillar[5]arenes: their Lewis acid catalyzed synthesis and host–guest property. J. Am. Chem. Soc. 130, 5022–5023. 10.1021/ja711260m. [DOI] [PubMed] [Google Scholar]
- Ogoshi T.; Yamagishi T. A.; Nakamoto Y. (2016) Pillar-shaped macrocyclic hosts pillar[n]arenes: new key players for supramolecular chemistry. Chem. Rev. 116, 7937–8002. 10.1021/acs.chemrev.5b00765. [DOI] [PubMed] [Google Scholar]
- Li Y.-F.; Li Z.; Lin Q.; Yang Y.-W. (2020) Functional supramolecular gels based on pillar[n]arene macrocycles. Nanoscale 12, 2180–2200. 10.1039/C9NR09532B. [DOI] [PubMed] [Google Scholar]
- Song N.; Lou X.-Y.; Ma L.; Gao H.; Yang Y.-W. (2019) Supramolecular nanotheranostics based on pillarenes. Theranostics 9, 3075–3093. 10.7150/thno.31858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoshi T.; Kakuta T.; Yamagishi T. A. (2019) Applications of pillar[n]arene-based supramolecular assemblies. Angew. Chem., Int. Ed. 58, 2197–2206. 10.1002/anie.201805884. [DOI] [PubMed] [Google Scholar]
- Chen L.; Cai Y.; Feng W.; Yuan L. (2019) Pillararenes as macrocyclic hosts: a rising star in metal ion separation. Chem. Commun. 55, 7883–7898. 10.1039/C9CC03292D. [DOI] [PubMed] [Google Scholar]
- Jie K.; Zhou Y.; Li E.; Huang F. (2018) Nonporous adaptive crystals of pillararenes. Acc. Chem. Res. 51, 2064–2072. 10.1021/acs.accounts.8b00255. [DOI] [PubMed] [Google Scholar]
- Li H.; Yang Y.; Xu F.; Liang T.; Wen H.; Tian W. (2019) Pillararene-based supramolecular polymers. Chem. Commun. 55, 271–285. 10.1039/C8CC08085B. [DOI] [PubMed] [Google Scholar]
- Kakuta T.; Yamagishi T. A.; Ogoshi T. (2018) Stimuli-responsive supramolecular assemblies constructed from pillar[n]arenes. Acc. Chem. Res. 51, 1656–1666. 10.1021/acs.accounts.8b00157. [DOI] [PubMed] [Google Scholar]
- Delavaux-Nicot B.; Ben Aziza H.; Nierengarten I.; Trinh T. M. N.; Meichsner E.; Chesse M.; Holler M.; Abidi R.; Maisonhaute E.; Nierengarten J. F. (2018) A rotaxane scaffold for the construction of multiporphyrinic light-harvesting devices. Chem. - Eur. J. 24, 133–140. 10.1002/chem.201704124. [DOI] [PubMed] [Google Scholar]
- Si W.; Xin P.; Li Z. T.; Hou J. L. (2015) Tubular unimolecular transmembrane channels: construction strategy and transport activities. Acc. Chem. Res. 48, 1612–1619. 10.1021/acs.accounts.5b00143. [DOI] [PubMed] [Google Scholar]
- Adiri T.; Marciano D.; Cohen Y. (2013) Potential 129Xe-NMR biosensors based on secondary and tertiary complexes of a water-soluble pillar[5]arene derivative. Chem. Commun. 49, 7082–7084. 10.1039/c3cc43253j. [DOI] [PubMed] [Google Scholar]
- Zafrani Y.; Kaizerman D.; Hadar M.; Bigan N.; Granot E.; Gosh M.; Alder-Abramovich L.; Patolsky F.; Cohen Y. (2018) Pillararene-based two-component thixotropic supramolecular organogels: complementarity and multivalency as prominent motifs. Chem. - Eur. J. 24, 15750–15755. 10.1002/chem.201801418. [DOI] [PubMed] [Google Scholar]
- Kaizerman-Kane D.; Hadar M.; Tal N.; Dobrovetsky R.; Zafrani Y.; Cohen Y. (2019) pH-responsive pillar[6]arene-based water-soluble supramolecular hexagonal boxes. Angew. Chem., Int. Ed. 58, 5302–5306. 10.1002/anie.201900217. [DOI] [PubMed] [Google Scholar]
- Joseph R.; Naugolny A.; Feldman M.; Herzog I. M.; Fridman M.; Cohen Y. (2016) Cationic pillararenes potently inhibit biofilm formation without affecting bacterial growth and viability. J. Am. Chem. Soc. 138, 754–757. 10.1021/jacs.5b11834. [DOI] [PubMed] [Google Scholar]
- Joseph R.; Kaizerman D.; Herzog I. M.; Hadar M.; Feldman M.; Fridman M.; Cohen Y. (2016) Phosphonium pillar[5]arenes as a new class of efficient biofilm inhibitors: importance of charge cooperativity and the pillar platform. Chem. Commun. 52, 10656–10659. 10.1039/C6CC05170G. [DOI] [PubMed] [Google Scholar]
- Gao L.; Li M.; Ehrmann S.; Tu Z.; Haag R. (2019) Positively charged nanoaggregates based on zwitterionic pillar[5]arene that combat planktonic bacteria and disrupt biofilms. Angew. Chem., Int. Ed. 58, 3645–3649. 10.1002/anie.201810314. [DOI] [PubMed] [Google Scholar]
- Yang K.; Chao S.; Zhang F.; Pei Y.; Pei Z. (2019) Recent advances in the development of rotaxanes and pseudorotaxanes based on pillar[n]arenes: from construction to application. Chem. Commun. 55, 13198–13210. 10.1039/C9CC07373F. [DOI] [PubMed] [Google Scholar]
- Ch’ng J.-H.; Chong K. L.; Lam L. N.; Wong J. J.; Kline K. A. (2019) Biofilm-associated infection by enterococci. Nat. Rev. Microbiol. 17, 82–94. 10.1038/s41579-018-0107-z. [DOI] [PubMed] [Google Scholar]
- Fernandez Guerrero M. L.; Goyenechea A.; Verdejo C.; Roblas R. F.; de Gorgolas M. (2007) Enterococcal endocarditis on native and prosthetic valves. Medicine 86, 363–377. 10.1097/MD.0b013e31815d5386. [DOI] [PubMed] [Google Scholar]
- Jansen K. U.; Girgenti D. Q.; Scully I. L.; Anderson A. S. (2013) Staphylococcus aureus clumping factor Aremains a viable vaccine target for prevention of S. aureus infection. Vaccine 31, 2723–2730. 10.1016/j.vaccine.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Algburi A.; Comito N.; Kashtanov D.; Dicks L. M. T.; Chikindas M. L. (2017) Control of biofilm formation: antibiotics and beyond. Appl. Environ. Microbiol. 83, e025908–16. 10.1128/AEM.02508-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman M.; Tanabe S.; Howell A.; Grenier D. C. (2012) Cranberry proanthocyanidins inhibit the adherence properties of Candida albicans and cytokine secretion by oral epithelial cells. BMC Complement. Altern. Med. 12, 6. 10.1186/1472-6882-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbuch K.; Benhamou R. I.; Levin L.; Stein R.; Fridman M. (2018) Increased degree of unsaturation in the lipids of antifungal cationic amphiphiles facilitates selective fungal cell disruption. ACS Infect. Dis. 4, 825–830. 10.1021/acsinfecdis.7b00272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



