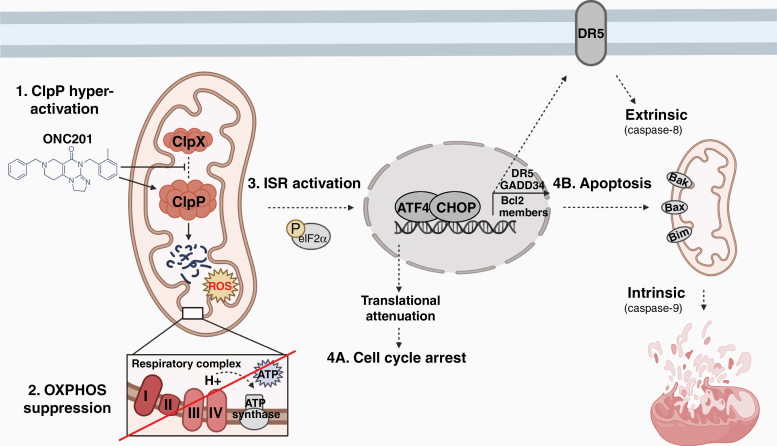
Fig. 1.
Anticancer mechanism of imipridones. Data from the existing literature indicates that imipridones exert their anticancer effects primarily by binding to and potently activating the mitochondrial Clp protease proteolytic subunit ClpP, causing ClpP to lose its dependence on the chaperone protein ClpX (Step 1). Hyper-active Clp protease then depletes its target substrates including components of the respiratory complex chain, most strongly complex I and II proteins (Step 2). In turn, OXPHOS is impaired and cellular ATP is depleted (Step 2). Mitochondrial structural damage and distress occurs, concomitant with the state of energy deprivation leading to integrated stress response (ISR) activation (Step 3). The ISR is relayed to the nucleus through an undefined mechanism, involving the typical (phospho-eIF2α-dependent) or atypical (phospho-eIF2α-independent) pathway. ISR activation causes global translational attenuation, including reduced levels of cyclin D1 leading to cell cycle arrest (Step 4A). In conditions of prolonged stress, ATF4 and CHOP are upregulated, and together these transcription factors increase the expression of their target genes including GADD34, which promotes further protein synthesis and stress (thus further activating the ISR); the TRAIL receptor DR5, which can promote TRAIL-mediated extrinsic cell death; and pro-apoptotic Bcl-2 family proteins, which promote the intrinsic, mitochondrial cell death program (Step 4B). Created with BioRender.com.