Table 1.
ONC201 Derivativesa
| Properties of imipridone compounds | ||||
|---|---|---|---|---|
| Imipridone | Structure | Properties | Putative targets | Ref |
| ONC201 |
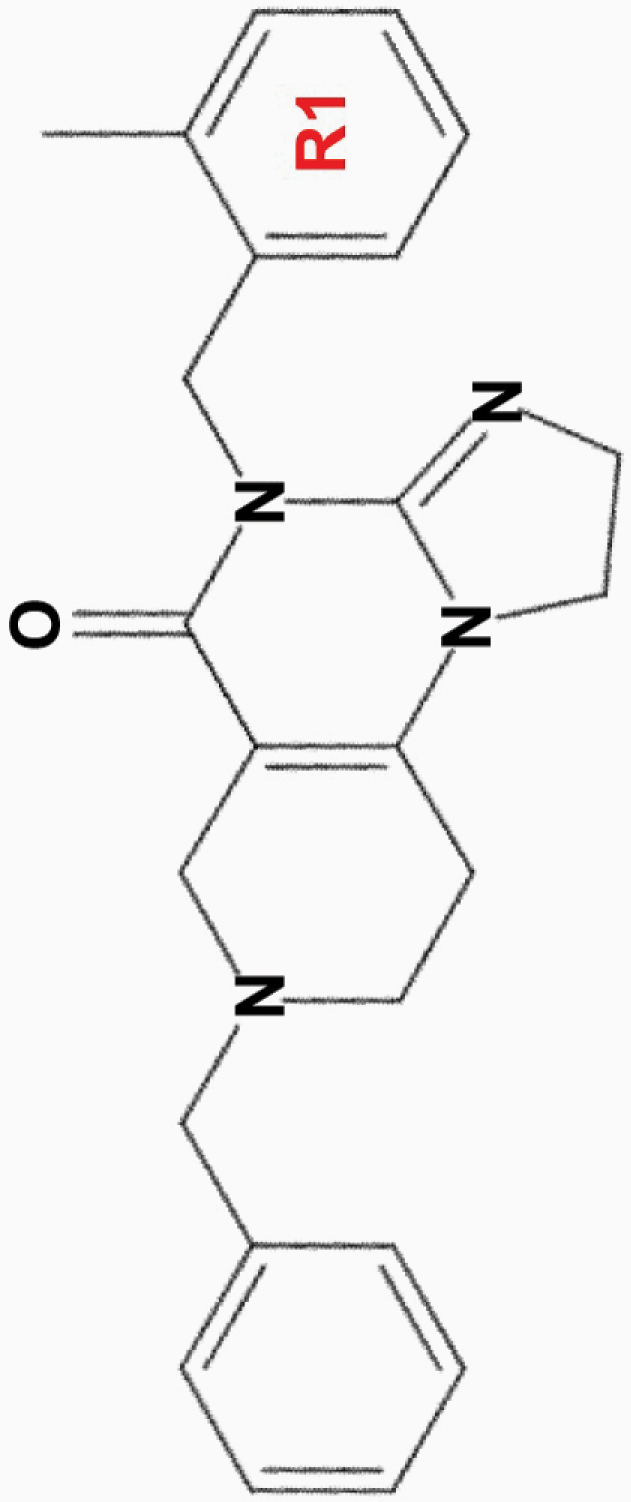
|
• Effective at micromolar (10 µM) concentrations | • Akt, ERK | 1,2,8,10,11,13,15–17 |
| • Anti-proliferative and pro-apoptotic effects | • TRAIL signaling | |||
| • Induces G2/M cell cycle arrest | • D(2) dopamine receptor | |||
| • Inhibits migration and invasion of colon cancer cells in vitro | ||||
| • ClpP | ||||
| • In phase 1/2 clinical trials for CNS, solid and hematologic cancers | • Androgen receptor (AR-V7) signaling (prostate cancer) | |||
|
ONC206
2,4-diF-benzyl |
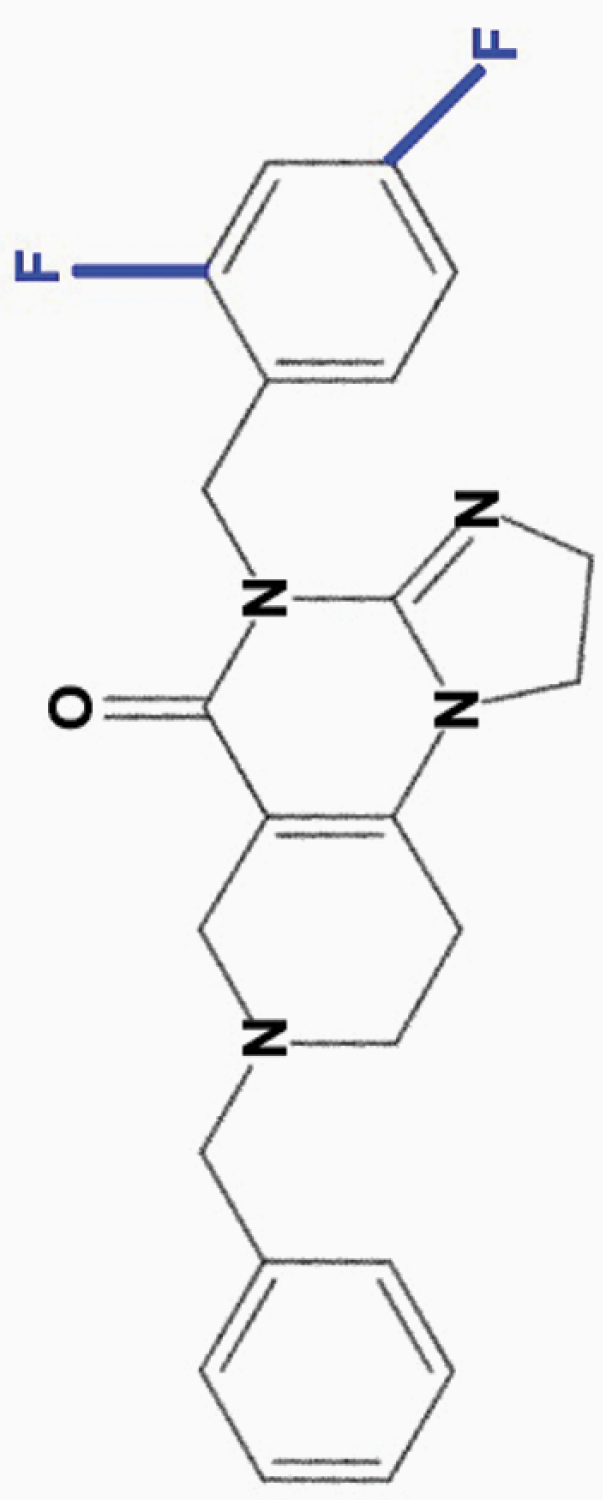
|
• Effective at nanomolar (50 nM) concentrations | 8,10 | |
| • Active against liver, lung, and kidney cancer cell lines | ||||
| • Prolonged efficacy compared to ONC201 and ONC212 | ||||
| • Stronger reduction of ATP levels compared to ONC201 | ||||
| • Inhibits migration and invasion of colon cancer cells in vitro | ||||
| • In phase 1 clinical trial for CNS cancers | ||||
|
ONC212
4-CF3-benzyl |
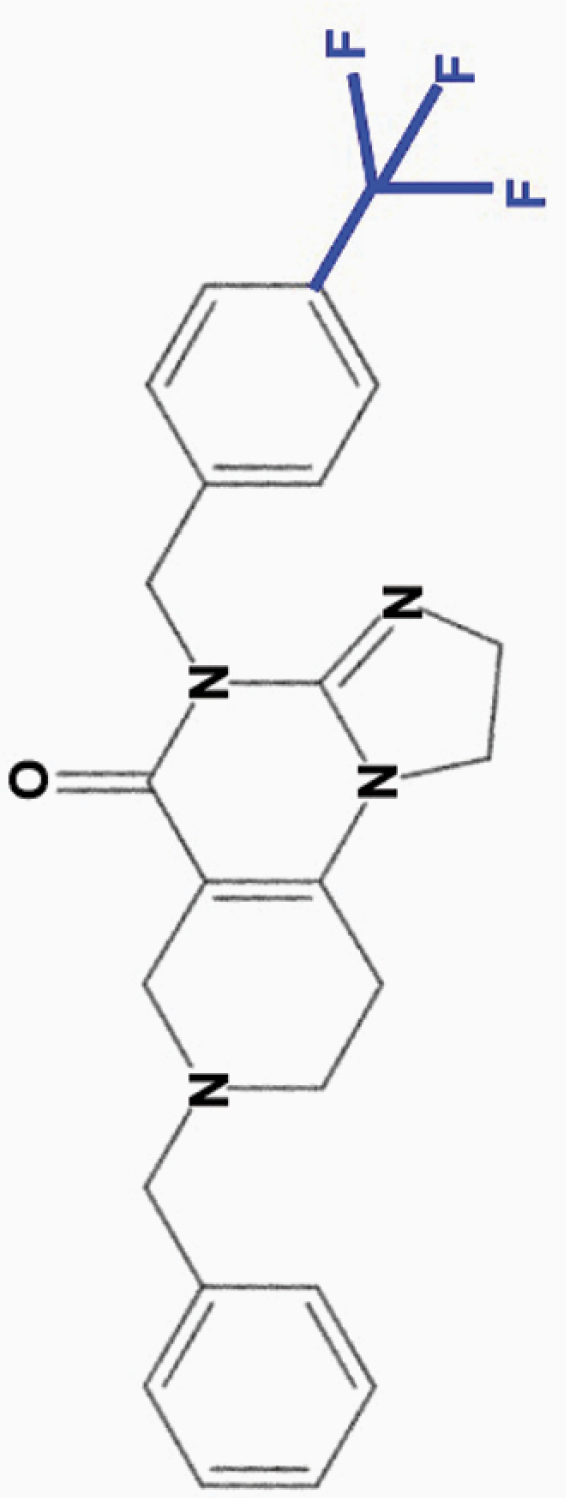
|
• Effective at nanomolar (10 nM) concentrations | • Binds ClpP with higher affinity than ONC201 | 8–12,17 |
| • Wider separation in toxicity between tumor and normal cells compared to ONC201 | ||||
| • Wider therapeutic index than ONC201; active against some, not all, ONC201-resistant cell lines | • Activates GPR132 and Gαq signaling (AML) | |||
| • Highly active against melanoma (including BRAF V600E mutant), prostate, breast, and thyroid cancer cell lines | ||||
| • Faster signaling effects than ONC201 and ONC206 | ||||
| • Shorter half-life in vivo, but prolonged mechanism | ||||
| • Induces cell cycle arrest at both G0/G1 and G2/M transition | ||||
| • Stronger inhibition of OXPHOS and reduction of ATP levels than ONC201 and ONC206 | ||||
| • Inhibits migration, but not invasion, of colon cancer cells in vitro | ||||
|
ONC213
3,4-diF-benzyl |
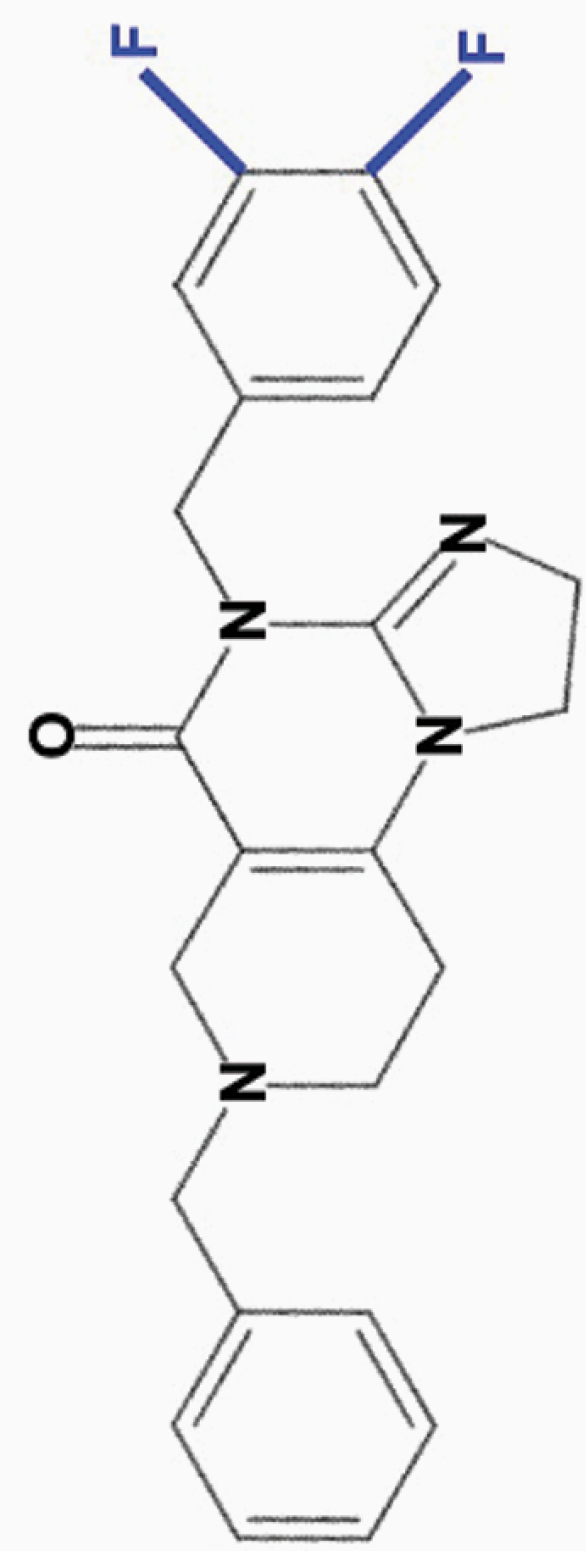
|
• Wide separation in toxicity between tumor and normal cells | 8 | |
|
ONC219
2,4-diCl-benzyl |
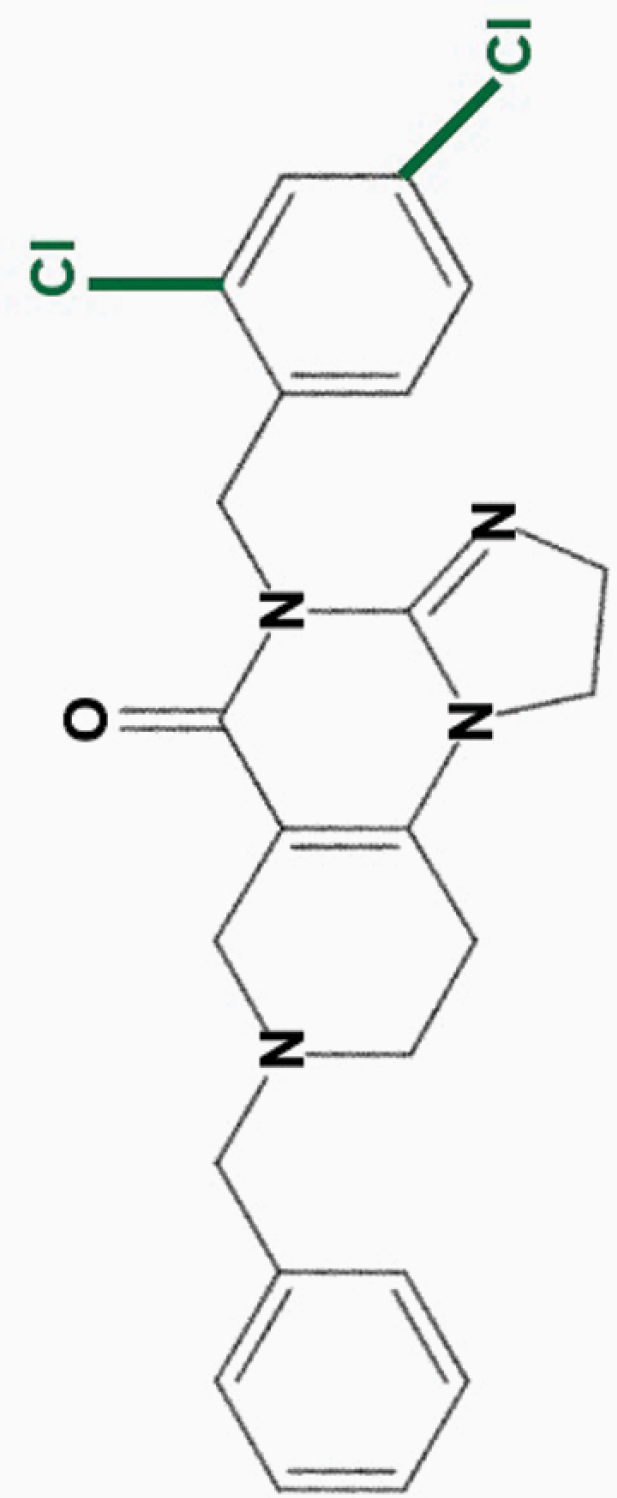
|
• Large therapeutic index in vitro | 8 | |
| • Potent efficacy against colorectal cancer cells | ||||
Abbreviation: Akt, protein kinase B; ERK, extracellular signal regulated kinase; TRAIL, tumor related apoptosis-inducing ligand; ClpP, caseinolytic protease P; ATP, adenosine triphosphate; AML, acute myeloid leukemia; OXPHOS, oxidative phosphorylation.
aONC201 is the parent compound of the imipridone family, and its modified derivatives share its core structure but harbor modifications to their peripheral benzyl moieties.8 Wagner and colleagues identified imipridone compounds harboring halogen (e.g. ONC212) or halide (e.g. ONC206, ONC209 [not shown]) substituents in the R1 position to be more potent than ONC201, and imipridones without a group at the benzyl substituent’s 2-position (e.g. ONC212, ONC213) to exhibit the widest separation between toxicity in tumor and normal cells.8 This table summarizes the properties and candidate biological targets of each imipridone. ONC201 derivatives are thought to share ONC201’s main biological targets, and as such, only additional targets specific to each compound are listed as their putative targets.
