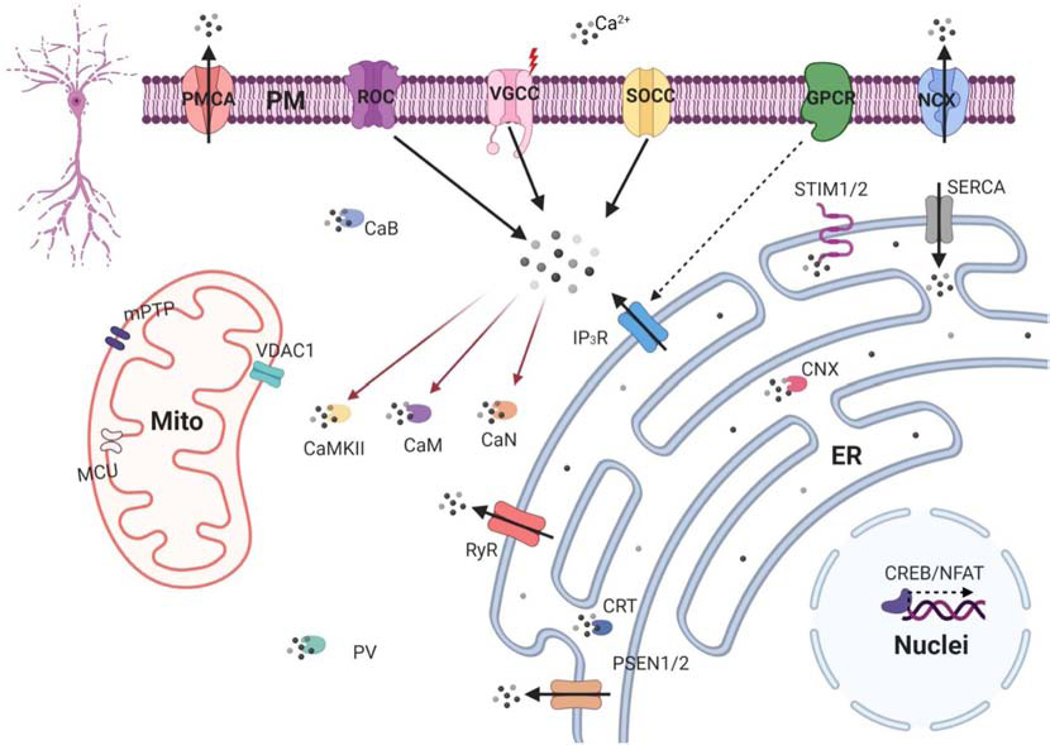
Figure 1. Neuronal calcium signaling.
Schematic representation of neuronal Ca2+ signaling. Main Ca2+ influx sources in plasma membrane (PM) are voltage-gated (VGCC), the ligand–operated (ROC) and the store-operated (SOCE) Ca2+ channels. Plasma membrane Ca2+ ATPase (PMCA) and the Na+/Ca2+ exchanger (NCX) extrude Ca2+ from cytosol into the extracellular space. Activation of metabotropic glutamate receptors (mGluR) stimulate Ca2+ mobilization from endoplasmic reticulum (ER) via inositol 1,4,5 - trisphosphate receptor (IP3R). Ca2+ also can be mobilized from ER via ryanodine receptors (RyR) amplifying cytoplasmic Ca2+ signals. Reuptake Ca2+ into the ER is operated by the ATPase SERCA. ER Ca2+ depletion is detected by Ca2+ sensors STIM1/2. To maintain the balance, Ca2+ is released from ER through passive leakage channels presenilins (PSEN1/2). The transmission of Ca2+-dependent signals to the nucleus occurs with the help of transcription factors cAMP response element-binding protein (CREB) and nuclear factor of activated T-cells (NFAT). The mitochondrial (mito) Ca2+ handling systems include mitochondrial Ca2+ uniporter (MCU), voltage-dependent anion channel type 1 (VDAC1), mitochondrial permeability transition pore (mPTP). Ca2+ concentration in cytosolic maintain with Ca2+-binding proteins: calbindin-28 (CaB), parvalbumin (PV) and calretinin (CaR), and inside ER with calreticulin (CRT) and calnexin (CNX). Ca2+-activated proteins include calmodulin (CaM), Ca2+/ calmodulin-dependent protein kinase type II (CAMKII) and Ca2+/calmodulin-dependent protein phosphatase calcineurin (CaN). Figure created with BioRender.com.