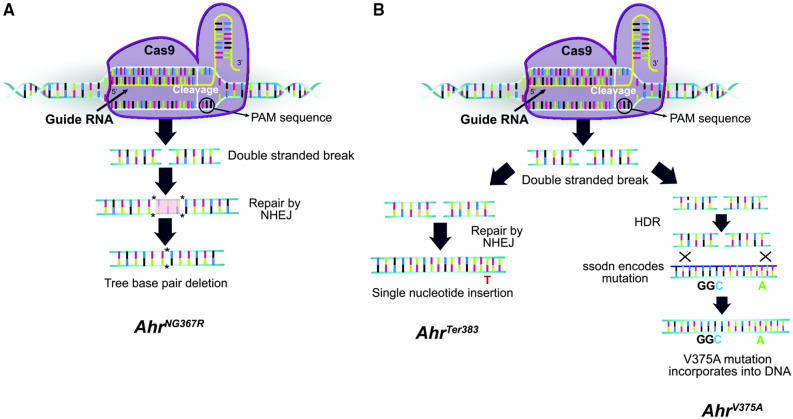
Figure 1.
Gene editing strategy for CRISPR-generated mutant mice. Alleles are shown in chronological order for which they were generated. The AhrNG367R allele was generated in an independent experiment using guide RNA no. 1, whereas AhrV375A and AhrTer383 alleles were generated on separate chromosomes in the same mouse during a second experiment using guide RNA no. 2. A, Proposed mechanism generating AhrNG367R allele. The Cas9 endonuclease incorporated guide RNA no. 1, directing the Cas9 protein to the Ahr locus in Ahrfx embryos. The Cas9 cleaved the DNA at the PAM sequence, inducing a double-stranded break. The cell recognized the double-stranded break and repaired the break using nonhomologous end joining (NHEJ). In this case, 3 bp were deleted resulting in the AhrNG367R mutation. B, Proposed mechanism generating the AhrV375A and AhrTer383 alleles. Guide RNA no. 2 directed Cas9 endonuclease in Ahrfx embryos. The Cas9 cleaved the DNA at the PAM site, inducing a double-stranded break. On 1 chromosome (left), the break was repaired through NHEJ, resulting in a single nucleotide insertion at the Ahr locus and the AhrTer383 mutation. On the second chromosome (right), the cell recognized homology with the ssODN. Homology directed repair (HDR) resulted in incorporation of the ssODN sequence at the Ahr locus, to yield the AhrV375A mutation. Both mutations occurred in one mouse, but each mutation occurred on a separate chromosome. The alleles were separated into distinct lines through breeding with wild-type B6 mice.