Abstract
Although COVID-19 affects the respiratory system, extrapulmonary manifestations frequently occur, including encephalopathy and liver damage. Here, we want to call attention to a possible connection between liver and brain dysfunctions, in which ammonia can play a role targeting astrocytes. Importantly, astrocyte dysfunction can produce future and/or long-term neurological consequences.
Keywords: COVID-19, Liver dysfunction, Brain dysfunction, Ammonia, Astrocytes
The coronavirus disease 2019 (COVID-19) pandemic has represented an unprecedented global health challenge. While SARS-CoV-2 infection affects mainly the respiratory tract, extrapulmonary manifestations frequently occur. Neurological symptoms that may affect COVID-19 patients range from mild symptoms, such as headache, dizziness, and loss of smell and taste, to psychiatric manifestations, stroke, encephalitis and encephalopathy (a mental status of confusion, disorientation, agitation and somnolence) (Wu et al., 2020). Although other viruses (Japanese encephalitis, Zika and even coronaviruses, including SARS-CoV) that cause neurological manifestations can directly infect the brain, the neuroinvasion by SARS-CoV-2 is currently under investigation (Song et al., 2021). Low levels of SARS-CoV-2 RNA have been reported in the post-mortem brain and cerebrospinal fluid of COVID-19 patients with encephalitis or encephalopathy, and imaging data has suggested neuroinflammatory mechanisms. As such, it is currently unclear as to whether the neurological symptoms associated with COVID-19 are directly caused by SARS-CoV-2 invasion of the brain or whether they result from systemic/peripheral illness, such as a dysregulated immune response and/or hepatic/metabolic dysfunction.
COVID-19 patients also often present liver damage, with a wide spectrum of clinical manifestations. However, as observed in the brain, while the presence of SARS-CoV-2 RNA has been detected in the liver, the hepatic injury incurred may also be the consequence of systemic inflammation, hypoxia, drug-induced liver disease or even reactivation of pre-existing liver diseases. Elevation of serum aspartate and alanine aminotransferase, hyperbilirubinemia and hypoalbuminemia are among the most common liver biochemistry abnormalities detected (Bloom et al., 2020); however, the evaluation of serum ammonia levels has been undervalued, despite reports of the occurrence of hyperammonemia in COVID-19 patients. It is important to note that liver dysfunctions are closely associated with brain dysfunctions, as observed in hepatic encephalopathy (HE) (Felipo, 2013). In this scenario, both inflammation and the accumulation of toxic metabolites, such as ammonia, play an important role.
1. Pathological events in sepsis-associated and hepatic encephalopathies
Encephalopathy is a neurological manifestation that can occur in both bacterial and viral infections. During sepsis, severe systemic inflammation produces a cytokine storm, which consists of a marked increase in pro-inflammatory mediators, such as tumor necrosis factor α (TNF-α), interleukins (IL-1β, IL-6, IL-12) and chemokines (Tranah et al., 2013). IL-6 is an important inducer of the acute phase response in the liver, inducing the production of C-reactive protein among other effects, in addition to potentially causing acute liver injury. In COVID-19, both hyperinflammatory scenarios and hepatic overactivation have been described and are marked by an increase in another acute phase protein particularly associated with viral infections, ferritin (Fara et al., 2020). It is important to note that liver failure, which frequently complicates sepsis, causes hyperammonemia in addition to potentiating systemic inflammation. As a consequence, both accumulated pro-inflammatory mediators and ammonia can synergically damage the blood-brain barrier (BBB), triggering a wide range of effects on neural cells and brain dysfunction.
2. Effects of systemic inflammation and hyperammonemia on astrocytes
Astrocytes participate in the formation and maintenance of the BBB, thus constituting a physical barrier to protect CNS. It is important to note that, during systemic inflammation and/or hyperammonemia, astrocytes undergo an activation process, which leads to morphological and functional changes that significantly and similarly participate in the pathogenesis of sepsis-associated encephalopathy (SAE) and HE (Bellaver et al., 2018; Bobermin et al., 2020; Tranah et al., 2013). Morphological remodeling during astrocyte reactivity can facilitate the disruption of BBB, resulting in infiltration of peripheral cytokines and immune cells. Importantly, in response to inflammation, astrocytes can acquire an activated state, leading to nuclear factor kappa B (NFκB)-dependent up regulation and the production and release of pro-inflammatory cytokines and chemokines (including TNF-α, IL-1β, IL-6 and monocyte chemoattractant protein-1, MCP-1), as well as the expression of genes associated with inflammatory responses, such as cyclooxygenase-2 (COX-2) and inducible nitric oxide (iNOS). Therefore, astrocytes perpetuate and self-amplify the inflammatory response within the CNS, acting as a bridge between systemic inflammation and neuroinflammation.
Importantly, the expression of the cyclin-dependent kinase inhibitor p21 gene in astrocytes is also modulated by systemic inflammation and hyperammonemia (Bobermin et al., 2020). p21 is potentially involved in NFκB activation and is an important cell cycle regulator that has been used as a marker of cellular senescence. Although senescence occurs biologically with aging, it may be prematurely induced by several stressors, especially during inflammatory conditions and hyperammonemia (Bellaver et al., 2018; Bobermin et al., 2020). Senescent cells, in turn, also acquire a pro-inflammatory secretory phenotype that characterize the phenomenon of inflammaging. It is important to note that the senescence of astrocytes, or “glial inflammaging”, has emerged as a potential mechanism associated with the cognitive dysfunctions observed in SAE and HE. Additionally, oxidative imbalance, downregulation in cytoprotective pathways and alterations in neurotransmission involving astrocytes can also contribute to the pathophysiology of encephalopathies.
Considering the multisystemic aspect of COVID-19 and the well-known cytokine storm produced during infection, it is reasonable to assume that similar events can occur in the CNS of COVID-19 patients. With particular regard to glial cells, autopsy data from COVID-19 patients have shown astroglial and microglial activation throughout the brain, accompanied by cytotoxic T cells infiltration, without current evidence of SARS-CoV-2 infection (Matschke et al., 2020). Moreover, increased plasma levels of glial fibrillary acidic protein and neurofilament light chain reinforce astroglial and neuronal commitment in COVID-19 (Kanberg et al., 2020). In addition to encephalopathy, an increasing number of studies have reported neuropsychiatric manifestations in COVID-19 patients. Alterations in the expressions of molecular markers associated with several neuropsychiatric disorders (such as depression, bipolar disorder, schizophrenia, alcohol dependence, among others) have also been identified in COVID-19 clinical samples and, interestingly, many of them are related to inflammatory signaling (Quincozes-Santos et al., 2021). Taken together, these data support roles for peripheral immune cells and inflammatory molecules and metabolites, produced by systemic inflammation and potentially by liver dysfunction, in the brain abnormalities observed in COVID-19 patients.
3. Concluding remarks
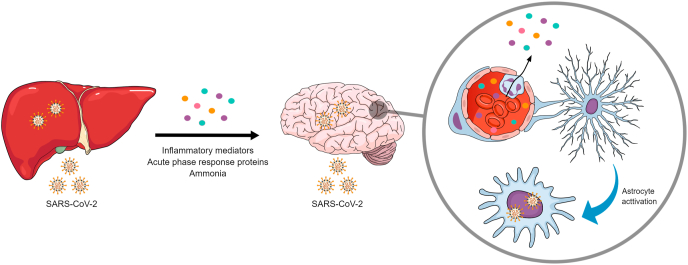
The need to assess the abnormalities associated with COVID-19 in different organs/systems in an integrative manner is becoming increasingly clear. Here, we call attention to the association between liver and brain dysfunctions, in which ammonia may play a crucial role, along with the production of cytokines and other inflammatory mediators (Fig. 1). Considering the versatility of astrocyte functions, it may be hypothesized that these cells are primary central targets of this relationship, receiving and integrating the peripheral signals to produce CNS responses. It is also important to point out that the occurrence of pre-existing liver diseases may aggravate and/or increase the risk of neurological manifestations in COVID-19, and that there may be a necessity for follow-up to observe hepatic function after infection. Finally, and just as importantly, while the encephalopathy associated with SARS-CoV-2 infection can be transient, cognitive deficits, neurodegenerative and psychiatric disorders might be long-term consequences of COVID-19.
Fig. 1.
The possible interplay between liver and brain dysfunctions in COVID-19. Patients with COVID-19 often present liver damage, but it is currently unclear whether this is a direct consequence of SARS-CoV-2 infection or secondary to other systemic events. The liver is able to respond to and participate in the inflammatory response caused by SARS-CoV-2. In addition, liver damage can impair ammonia metabolism, increasing the circulating concentrations of this neurotoxic metabolite. Neurological symptoms frequently occur in COVID-19 patients, but the possibility of neuroinvasion by SARS-CoV-2 is also under investigation. However, the harmful effects of peripheral inflammatory mediators and ammonia on astrocyte functions are well established and are known to trigger morphological and neurochemical changes in these cells, which actively participate in the pathophysiology of encephalopathy. Colored circles represent inflammatory mediators, acute phase response proteins and ammonia, and the circle box displays astrocyte activation promoted by these molecules.
Funding
The authors of this study are supported by Universidade Federal do Rio Grande do Sul (UFRGS), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS).
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author’s contributions
LDB and AQS conceived, wrote and revised the manuscript. LDB and AQS designed and created the figure.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors are supported by the Universidade Federal do Rio Grande do Sul (UFRGS), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS).
References
- Bellaver B., Dos Santos J.P., Leffa D.T., Bobermin L.D., Roppa P.H.A., da Silva Torres I.L., Gonçalves C.-A., Souza D.O., Quincozes-Santos A. Systemic inflammation as a driver of brain injury: the astrocyte as an emerging player. Mol. Neurobiol. 2018;55:2685–2695. doi: 10.1007/s12035-017-0526-2. [DOI] [PubMed] [Google Scholar]
- Bloom P.P., Meyerowitz E.A., Reinus Z., Daidone M., Gustafson J., Kim A.Y., Schaefer E., Chung R.T. Liver biochemistries in hospitalized patients with COVID-19. Hepatology. 2020 doi: 10.1002/hep.31326. [DOI] [PubMed] [Google Scholar]
- Bobermin L.D., Roppa R.H.A., Gonçalves C.-A., Quincozes-Santos A. Ammonia-induced glial-inflammaging. Mol. Neurobiol. 2020;57:3552–3567. doi: 10.1007/s12035-020-01985-4. [DOI] [PubMed] [Google Scholar]
- Fara A., Mitrev Z., Rosalia R.A., Assas B.M. Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines. Open Biol. 2020;10:200160. doi: 10.1098/rsob.200160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felipo V. Hepatic encephalopathy: effects of liver failure on brain function. Nat. Rev. Neurosci. 2013;14:851–858. doi: 10.1038/nrn3587. [DOI] [PubMed] [Google Scholar]
- Kanberg N., Ashton N.J., Andersson L.-M., Yilmaz A., Lindh M., Nilsson S., Price R.W., Blennow K., Zetterberg H., Gisslén M. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020;10 doi: 10.1212/WNL.0000000000010111. 1212/WNL.0000000000010111. [DOI] [PubMed] [Google Scholar]
- Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., Mushumba H., Fitzek A., Allweiss L., Dandri M., Dottermusch M., Heinemann A., Pfefferle S., Schwabenland M., Sumner Magruder D., Bonn S., Prinz M., Gerloff C., Püschel K., Krasemann S., Aepfelbacher M., Glatzel M. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quincozes-Santos A., Rosa R.L., Tureta E.F., Bobermin L.D., Berger M., Guimarães J.A., Santi L., Beys-da-Silva W.O. COVID-19 impacts the expression of molecular markers associated with neuropsychiatric disorders. Brain, Behavior, & Immunity - Health. 2021;11:100196. doi: 10.1016/j.bbih.2020.100196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E., Zhang C., Israelow B., Lu-Culligan A., Prado A.V., Skriabine S., Lu P., Weizman O.-E., Liu F., Dai Y., Szigeti-Buck K., Yasumoto Y., Wang G., Castaldi C., Heltke J., Ng E., Wheeler J., Alfajaro M.M., Levavasseur E., Fontes B., Ravindra N.G., Van Dijk D., Mane S., Gunel M., Ring A., Kazmi S.A.J., Zhang K., Wilen C.B., Horvath T.L., Plu I., Haik S., Thomas J.-L., Louvi A., Farhadian S.F., Huttner A., Seilhean D., Renier N., Bilguvar K., Iwasaki A. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021;218 doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranah T.H., Vijay G.K.M., Ryan J.M., Shawcross D.L. Systemic inflammation and ammonia in hepatic encephalopathy. Metab. Brain Dis. 2013;28:1–5. doi: 10.1007/s11011-012-9370-2. [DOI] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.