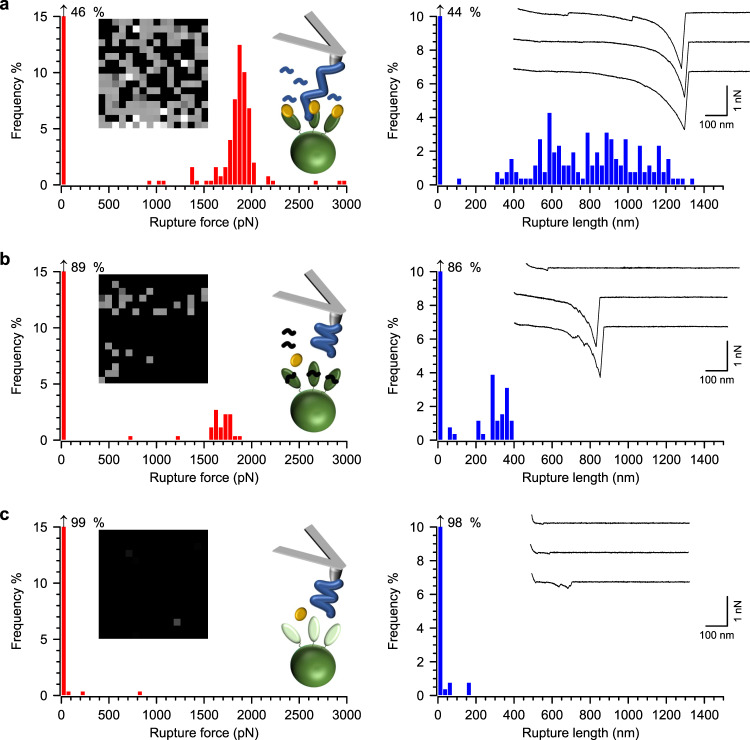
Fig. 6. A dock, lock, and latch (DLL) interaction might be the key for a highly stable vWF-vWbp-ClfA complex.
a ClfA+ S. aureus cells were first treated for 15 min with a peptide with a random sequence and then with recombinant vWbp before being probed with vWF-modified AFM tips. Data for a representative cell are shown. On the left are histograms of rupture forces with insets showing the respective adhesion maps (500 × 500 nm, 16 × 16 pixels, gray scale = 0–3 nN, each dot represents a binding event) and a cartoon in the top graph illustrates the experimental setup. The random peptide is illustrated by blue curled lines. On the right are shown histograms of the rupture lengths with insets showing three representative curves. b Data for ClfA+ S. aureus cells treated for 15 min with a peptide with the Fg γ-chain C-terminal sequence that binds to ClfA (black curled lines). vWbp was then added for 15 mins and the cells probed with vWF-modified tips. c ClfAPY cells were treated with recombinant vWbp and then probed with vWF-modified AFM tips.