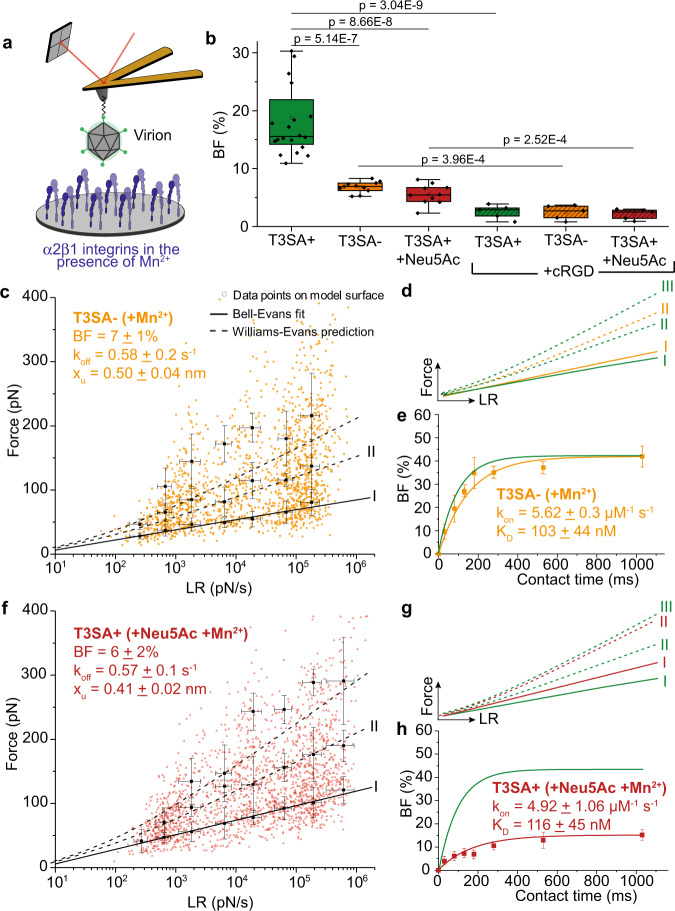
Fig. 4. Binding of the reovirus σ1 attachment protein to sialic acid alters β1 integrin interaction.
The effect of a point mutation in the SA-binding site of σ1 protein (c–e) or addition of exogenous α-SA (f–h) on reovirus-α2β1 integrin interactions was determined using model surfaces. a Schematic depicts the experimental set up. All experiments were conducted in the presence of Mn2+. b Box plot shows BF between T3SA+ or T3SA- virions and integrins quantified using AFM under the conditions shown. The horizontal line within the box indicates the median, boundaries of the box indicate the 25th and 75th percentile, and whiskers indicate the highest and lowest values. An open square within each box indicates the mean. c DFS plot shows interaction forces between T3SA- and integrins. d Comparison of forces required to rupture bonds between integrins and T3SA+ (green) or T3SA- (yellow). e BF plotted as a function of contact time shows comparable kon and KD values for T3SA+ (green) and T3SA- (yellow) interactions with integrins. f–h DFS plot (f) and kinetic on-rate measurements (h) show differences in T3SA+ interactions with β1 integrins in the presence (red) and absence (green) of α-SA (1 mM Neu5Ac) (g). Error bars indicate s.d. of the mean. All data are representative of N = 5 independent experiments. P values were determined by two-sample t-test using Origin.