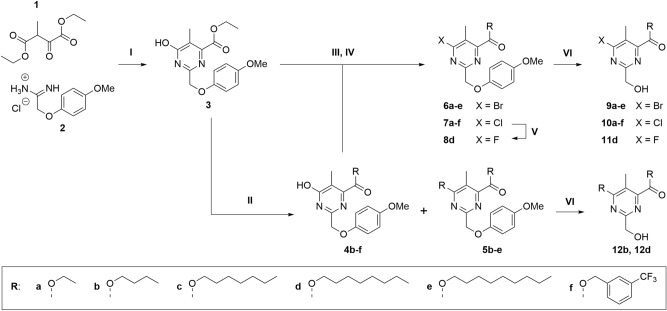
Figure 2.
Synthesis and derivatization routes of sets I and II of 2,4,5,6-tetrasubstituted pyrimidines. Conditions: (I) TEA, EtOH, reflux, 2.5 h, 31%; (II) alcohol, H2SO4 (cat.), 100 °C, 3–48 h, 18–58%; (III) POBr3, DMF, MW 90 °C, 10–15 min, 41%–quant.; (IV) POCl3, DMF, MW 90 °C, 10–15 min, 72%–quant.; (V) KF, TBAB, sulfolane, MW 150 °C, 2 h, 35%; (VI) CAN, MeCN/H2O, − 15 °C, 10 min, 12–75%.