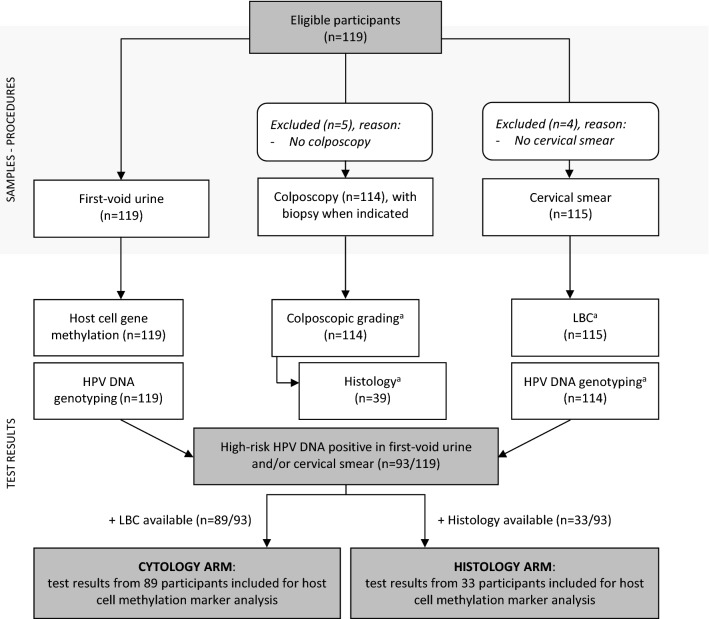
Figure 1.
Flow diagram for the inclusion of study participants, samples, and medical records. Results from 119 participants were used for HPV DNA genotyping in first-void urine and cervical samples. Test results from high-risk HPV DNA positive women (in first-void urine and/or cervical smear, n = 93) were used to examine methylation marker performance; using either cytology results (CYTOLOGY ARM), or histology outcomes (HISTOLOGY ARM) as reference. aWhen unavailable at D0 (day of study visit, i.e. first-void urine collection), the HPV DNA genotyping, liquid based cytology (LBC), colposcopy, and/or histology results from D0 ± 3 months were included for data analysis instead. Thus, three additional HPV DNA genotyping results were included, as well as four LBC and colposcopy, and six histology results.