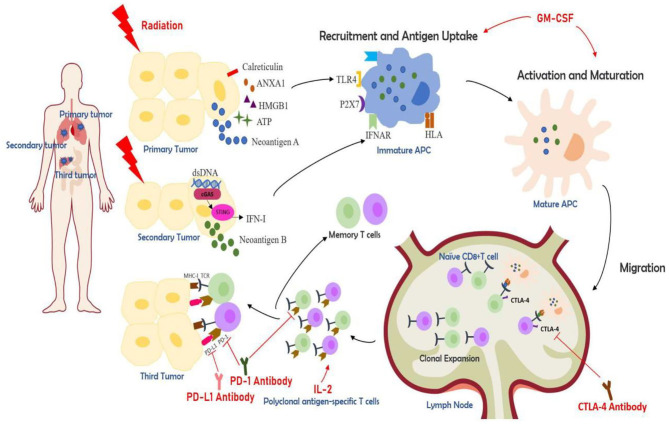
Figure 1.
The partial mechanisms of multisite radiotherapy combined with immune checkpoint inhibitors (ICIs) and biological response modifiers (GM-CSF or IL-2). Radiotherapy induces immunogenic cell death (ICD), which exposes and releases danger-associated molecular patterns (DAMPs) like calreticulin, HMGB1, ATP, ANAX1, and similar (12). cGAS-STING pathway is activated by the cytolytic double-strand DNA and results in the release of IFN-I (10). Radiation can also generate tumor neoantigens. Multisite radiotherapy can overcome the insufficient tumor-associated antigen (TAA) exposure caused by tumor heterogeneity (7). ICDs can recruit antigen-presenting cells (APC) like dendritic cells (DC). APCs can take up antigens and further be activated, which can be augmented by GM-CSF. DCs then migrate to lymph nodes, presenting antigens to T cells and prime a cytotoxic T lymphocyte (CTL)-mediated immune activation (26, 27). The activated CTLs initiate clonal proliferation and then travel to the irradiated lesions or distant tumor sites, exerting killing effects. The cytokine IL-2 is essential for the proliferation, differentiation, and survival of T cells (28). CTLA-4 antibody, PD-1 antibody, and PD-L1 antibody, known as ICIs, can increase CTL activation and boost the synergistic anti-tumor effects.