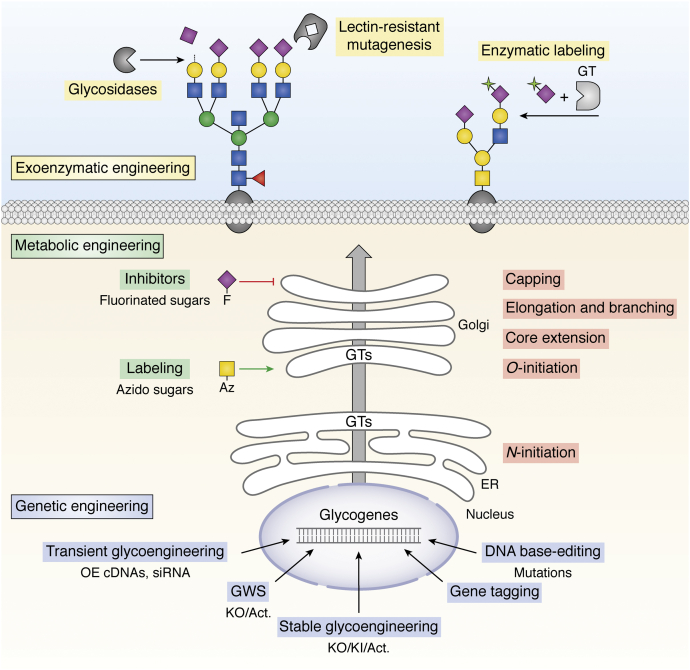
Figure 1.
Overview of glycoengineering strategies. Basic principles for approaches available to modulate the cellular glycosylation processes and the glycome are illustrated. Extracellularly, glycans may be modulated by more or less selective endo-/exo-glycosidases (sialidases, galactosidases, PNGase, etc.) (117), and chemoenzymatic labeling methods utilizing, e.g., glycosyltransferases (GTs) may be applied to install natural or unnatural substrates on cell surface glycans (6, 337). Use of cytotoxic lectins often in combination with mutagenesis may enable selection of mutant cells with loss/gain of distinct glycosylation features (10, 338). A growing number of unnatural sugar mimetics can be applied for metabolic engineering (6, 23), including glycosylation inhibitors (i.e., fluorinated sugar analogues) (235, 339) or functionalized sugars (i.e., azido, Az, sugars) that enable conjugation chemistries for use in glycan imaging or reprogramming of their interactions (20, 160). Genetic engineering of glycosylation may be performed by overexpression (OE) of GTs using cDNA plasmid transfection and/or siRNA for silencing of endogenous GTs (6). More extensive and stable glycoengineering takes advantage of precise gene engineering for combinatorial KO/KI/Act (activation) of GTs, and this strategy is the main focus of this review. Genome-wide KO/Act screens (GWS) may be used for discovery and dissection of GTs and other genes affecting glycosylation (Table 1), and endogenous GTs may be mutated, e.g., to mimic disease mutations or enable use of unique substrates, or tagged, e.g., by insertion of antibody tags or fluorescent proteins (169).