Figure 21.
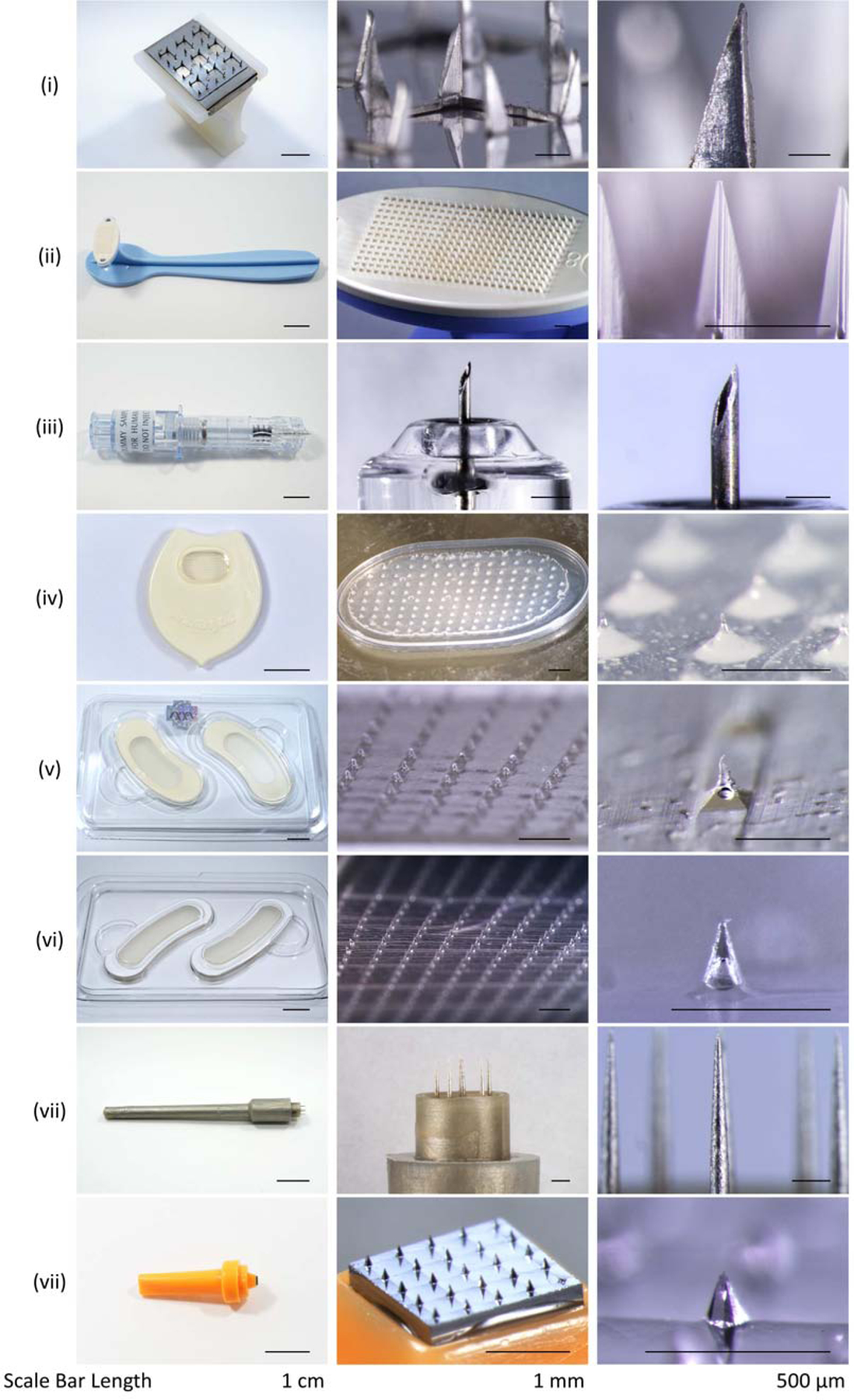
Representative examples of microneedle products approved for medical use or sold as cosmetics. (i) Sterile Multipuncture Device, Organon Teknika Corporation (Durham NC, USA). (ii) Microchannel Skin System, 3M (St. Paul, MN, USA). (iii) Soluvia microinjection system, BD (Franklin Lakes, NJ, USA). (iv) MicroHyala, CosMED Pharmaceutical (Kyoto, Japan). (v) Wellage Hyaluronic Acid Micro Needle Patch, Hugel (Chuncheon, South Korea). (vi) Reviewcell Snow White Hyaluronic Sheet, Soya Greentech (Seoul, South Korea). (vii) Dermastamp, Dermaroller (Wolfenbüttel, Germany). (viii) Liteclear Acne Treatment System, Nanomed Skincare (Cupertino, CA, USA).
