Figure 1.
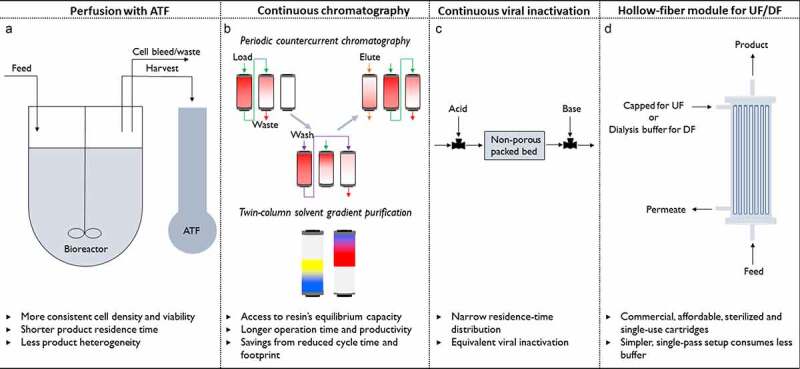
A subset of proposed technologies for continuous biopharmaceutical manufacturing. (a) Perfusion cell culture with alternating tangential flow filtration (ATF) for cellular retention, (b) 3-column periodic countercurrent chromatography for product capture44 and a two-column solvent gradient purification method for product polishing,45 (c) a continuous packed bed viral inactivator,46,47 and (d) hollow fiber dialysis module for continuous ultrafiltration48 and diafiltration.49
