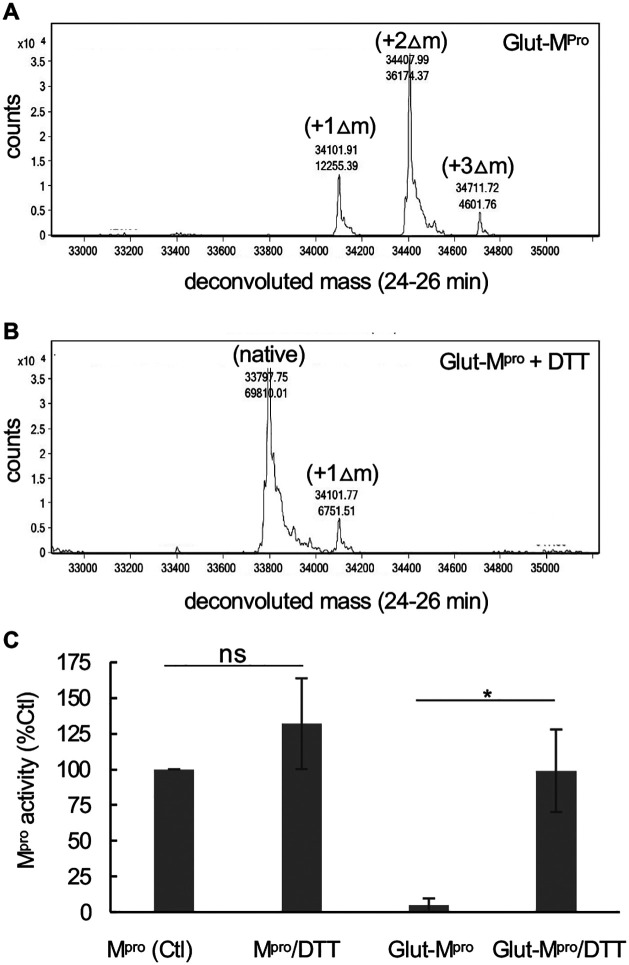
Figure 2: Inhibition of Mpro by glutathionylation can be reversed with reducing agent.
Mpro was glutathionylated at pH 7.5 with 10 mM GSSG as described in the Materials and Methods and the extent of glutathionylation was determined by RP/HPLC/MALDI-TOF using a 2% acetonitrile gradient as described in materials and Methods. (A,B) Deconvoluted masses obtained by protein deconvolution of the Mpro peak (eluting between 24 and 26 min) for (A) 3 μg (5 μL injection) purified glutathionylated Mpro and (B)) 3 μg (5 μL injection) glutathionylated Mpro after a 30 min treatment with 10 mM DTT. Shown above each peak is the molecular mass (top number) and the abundance (bottom number) found by protein deconvolution. The native, monoglutathionylated (+Δ1), diglutathionylated (+Δ2), and triglutathionylated (+Δ3) Mpro, are indicated in the figures. (C) Mpro activity (1 μM final enzyme) for native and glutathionylated Mpro preparations after a 30-min incubation in the absence or presence of 10 mM DTT. Mpro activity for control in (C) and was 4.95 +/− 1.2 μM/min/mg and percent activity for the different conditions was normalized to their respective controls. The values shown are the average and standard deviation from three separate experiments (n=3) (* = p-value < 0.05, paired students t-test, ns = not significant).