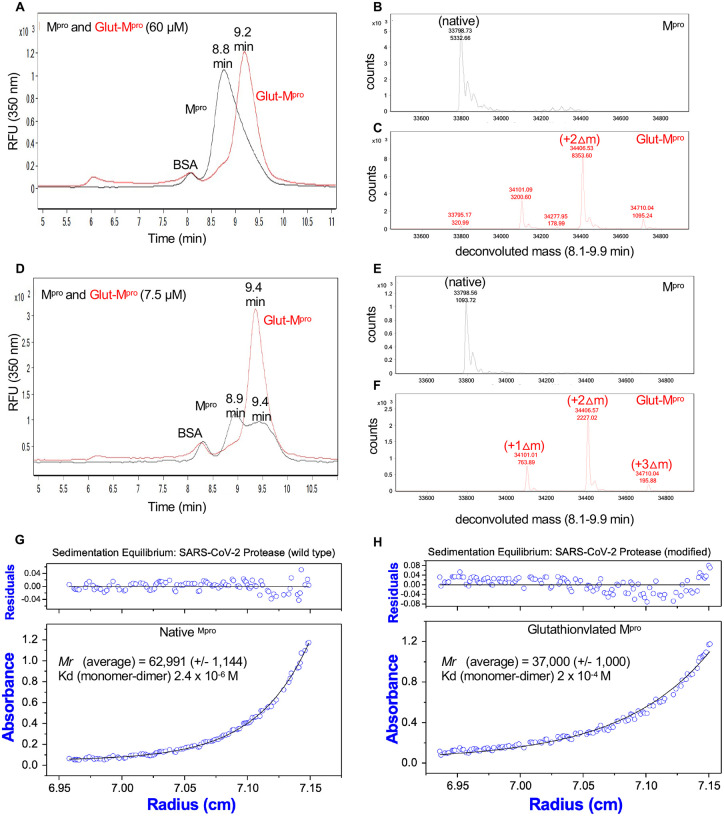
Figure 3: Size exclusion chromatography and equilibrium analytical ultracentrifugation of Mpro and glutathionylated Mpro indicates glutathionylated Mpro behaves as a monomer.
(A,D) Mpro and glutathionylated Mpro were analyzed by SEC3000/MALDI-TOF. (A) Overlay of the chromatograms for 60 μM each of Mpro (black line) and glutathionylated Mpro (red line) and (D) 7.5 μM each of Mpro (black line) and glutathionylated Mpro (red line) by monitoring the intrinsic protein fluorescence (excitation 276 nm, emission 350 nm). Glutathionylated Mpro was made with 10 mM GSSG at pH 7.5 for 2–2.5 hours as described in Materials and Methods. (C,D) Protein deconvolution profiles for (B) native Mprov and (C) glutathionylated Mpro that were run as shown in (A). (E,F) Protein deconvolution profile for (E) native Mpro and (F) glutathionylated Mpro that were run as shown in (D). Shown above each peak are the molecular mass (top number) and the abundance (bottom number) found by protein deconvolution. The earlier eluting peak at 8.5 min is cm-BSA, which was used as a carrier in the runs of Mpro to help prevent potential non-specific losses of protein during the run. (G,H) Equilibrium analytical ultracentrifugation of (G) Mpro and (H) glutathionylated Mpro at 0.63 mg ml−1 (18 μM) in 50 mM tris buffer pH 7.5, 2 mM EDTA, and 100 mM NaCl. The absorbance gradients in the centrifuge cell after the sedimentation equilibrium was attained at 21,000 rpm are shown in the lower panels. The open circles represent the experimental values, and the solid lines represent the results of fitting to a single ideal species. The best fit for the data shown in (G) yielded a relative molecular weight (Mr) of 62,991 +/− 1144 and a Kd for dimerization of 2.4 μM and that shown in (H) yielded a molecular weight of 37,000 +/− 1000 and a Kd for dimerization of 200 μM. The corresponding upper panels show the differences in the fitted and experimental values as a function of radial position (residuals). The residuals of these fits were random, indicating that the single species model is appropriate for the analyses.