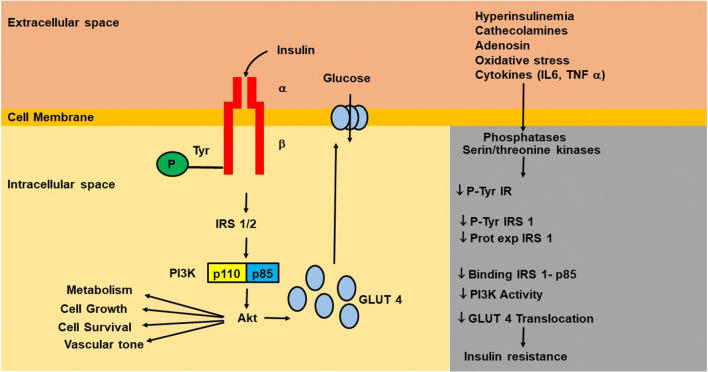
Fig. 2.
Schematic representation of early steps of insulin signaling, and the principal mechanisms that account for insulin resistance. Binding of insulin to the α-subunit of its own receptor induces the autophosphorylation of tyrosine residues of β-subunit which, in turn, induces the tyrosine phosphorylation of IR substrates (IRS), IRS-1, and IRS-2. The binding of phosphorylated IRS1/2 to the regulatory subunit p85 of phosphoinositide-3 kinase (PI3K) activates the catalytic subunit p110, which, in turn, phosphorylates/activates the serine/threonine kinase Akt (called also PKB), which stimulates the glucose uptake through the translocation of the major glucose transporter GLUT-4 to the plasma membrane. In addition, AKT promotes cell survival and cell growth, and is involved in the regulation of vascular tone and metabolic homeostasis. Insulin resistance evoked by oxidative stress, neuro-hormonal stimulation, hyperinsulinemia, and etc. may be due to defect(s) of downstream signaling components, such as receptor structure, number, binding affinity, and/or signaling capacity. P: phosphorylation, Tyr: tyrosine, IR. insulin receptor, IL-6: interleukine-6, Pro Exp: protein expression, TNF: tumor necrosis factor