Figure 1.
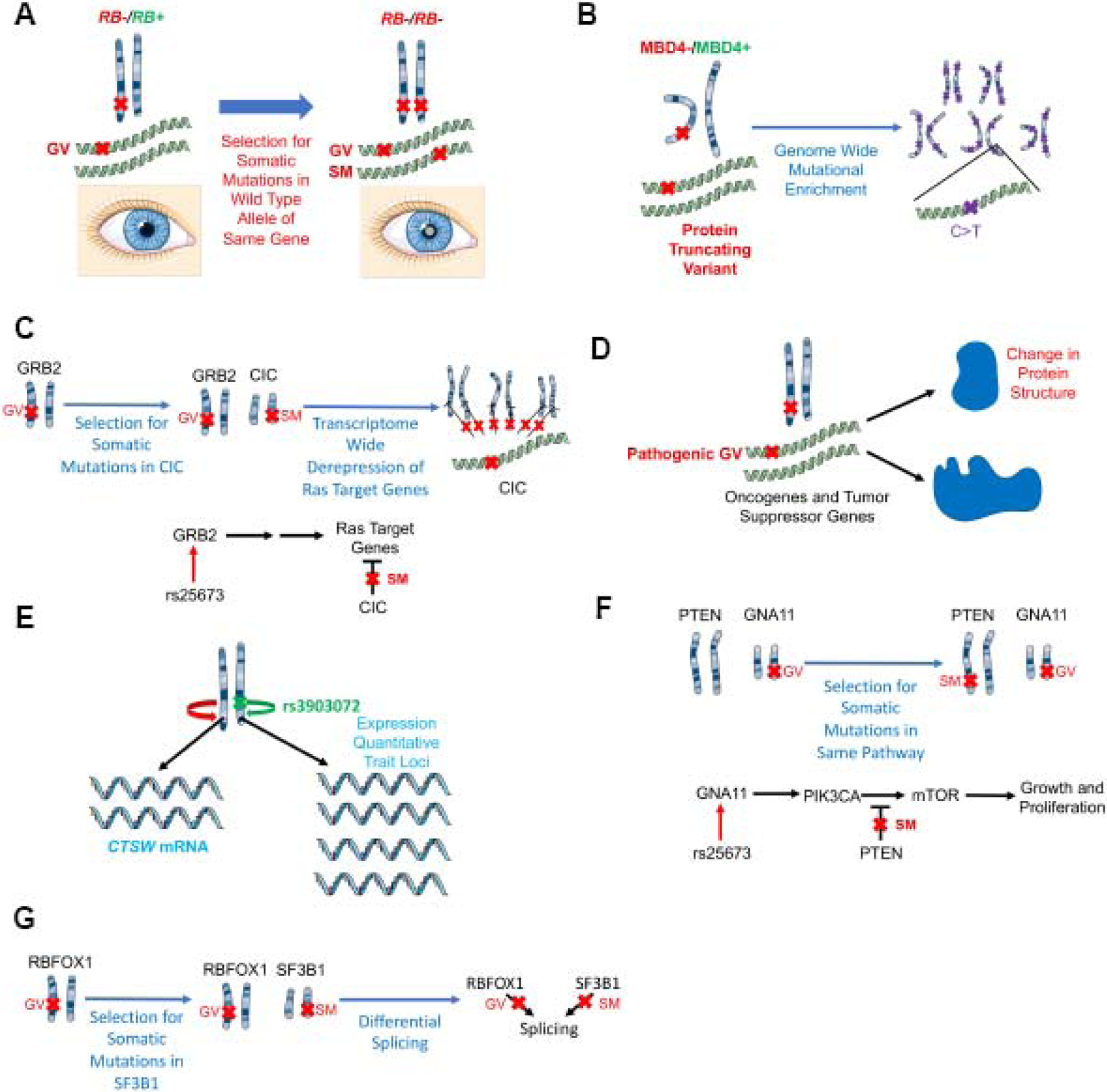
Molecular mechanisms by which germline variants may associate with outcome.
(A) Germline variants increase the likelihood of a somatic mutation in the wild type allele of the same gene: a germline variant in RB1 increases the likelihood of a somatic mutation in the other allele of RB1 leading to retinoblastoma (shown here by the clinical sign of leukocoria).
(B) Germline variants increase specific types of genome wide mutations: germline variants in MBD4 are associated with elevated C>T somatic mutations at CpG dinucleotides, consistent with the role of MBD4 in correcting G:T mismatches.
(C) Germline variants are associated with specific transcriptome alterations: a germline variant in the Ras signaling gene GRB2 is associated with upregulation of Ras target genes.
(D) Molecularly, germline variants may alter the function of tumor suppressor genes or oncogenes through amino acid changes.
(E) Germline variants act as expression quantitative trait loci (eQTL) in cis: a germline variant in the putative regulatory element of the tumor suppressor gene CTSW is associated with increased expression of this tumor suppressor gene.
(F) Germline variants increase the likelihood of additional somatic mutations in the same pathway: germline variants in GNA11, an activator of mTOR signaling, promote the acquisition of somatic mutations in PTEN, a tumor suppressor that suppresses mTOR signaling.
(G) Germline variants perturb splicing machinery: germline variants in RBFOX1, an RNA binding protein involved in splicing, are associated with increased somatic mutations in SF3B1, another component of the spliceosome, leading to varied splicing patterns.
