Abstract
BACKGROUND,
The utility of Subjective Cognitive Decline (SCD) as an indicator of preclinical AD is overshadowed by its inconsistent association with objective cognition.
OBJECTIVE,
This study examines if manipulations of SCD measurement affect its association with early cognitive dysfunction characteristic of preclinical AD.
METHODS,
Cognitively healthy older adults (n=110) completed SCD questionnaires that elicited complaints in general, compared to 5 years ago (retrospective SCD) and compared to their peers (age-anchored SCD) in binary and Likert scales. Outcome cognitive tasks included an associative memory task (Face-Name Test), a visual short-term memory binding task (STMB test), and a clinical neuropsychological list learning test (Selective Reminder Test).
RESULTS,
SCD complaints, when compared to age matched peers (age-anchored SCD) were endorsed less frequently than complaints compared to 5 years ago (retrospective SCD) (p<.01). In demographically adjusted regressions, age-anchored ordinal-rated SCD was associated with short term memory binding (β=−.22, p=.040, CI=−.45,−.01), associative memory (β=−.26, p=.018, CI=−.45,−.06) and list learning (β=−.31, p=.002, CI=−.51,−.12). Retrospective and general ordinal-rated SCD associated with associative memory (β=−.25, p=.012, CI=−.44,−.06; β=−.29, p=.003, CI=−.47,−.10) and list learning only (β=−.25, p=.014, CI=−.45,−.05; β=−.28, p=.004, CI=−.48,−.09).
CONCLUSION,
Ordinal Age-anchored SCD appears better suited than other SCD measurements to detect early cognitive dysfunction characteristic of preclinical AD.
Keywords: Subjective Cognitive Decline, cognitive dysfunction, neuropsychological tests, task-specific factors, measurement, preclinical Alzheimer’s disease
Introduction
In an attempt to improve the therapeutic window for Alzheimer’s disease (AD) efforts have focused on identifying individuals when they are in the earliest stage of AD which precedes overt cognitive and functional impairment [1]. This preclinical stage is defined by the presence of AD biomarkers, conceptualized within the ATN (amyloid, tau, neurodegeneration) framework, in the absence of clinically impaired cognitive function [2]. However, identifying individuals through biomarker testing is invasive, costly and hard to access. Moreover, biological gold standards of pre-clinical AD (e.g., amyloid positivity) do not provide direct information about the clinical transition to AD dementia; indeed, a significant proportion of individuals who meet neuropathological criteria for AD upon autopsy were clinically normal in life [3].
SCD is the subjective perception that one’s cognition has declined, before such decline is evident on standard diagnostic testing. SCD, hypothesized to precede Mild Cognitive Impairment, was suggested as a marker of preclinical Alzheimer’s disease (AD) decades ago [4, 5]. Recently, a series of studies have linked SCD to brain-based AD biomarkers such as amyloid and tau accumulation and brain degeneration, and various longitudinal studies have shown that increased SCD increases the risk of future cognitive impairment and dementia [6–11]. In contrast to biomarkers, SCD is non-invasive, inexpensive, and easily obtainable. Perhaps most importantly, it may be the first indication of a transition to a symptomatic stage of disease. Although emerging work is reinforcing the potential use of SCD as a marker of preclinical AD [12], there are important questions about SCD that first need to be addressed in order to elucidate SCD’s true utility as a harbinger of preclinical AD.
SCD is a complex, multidimensional construct influenced by several factors that can hamper its reflection of the pathological process underlying preclinical AD. Such factors can be grouped broadly into person-specific factors (i.e., individuals’ characteristics such as mood, personality, etc.), and task-specific factors (i.e., the specific way SCD is measured), the latter being the focus of the current paper. As highlighted by Rabin et al [13], a variety of SCD measures have been used across different studies, with relatively little attention being paid to the role that such variability plays in producing inconsistent associations between SCD and outcomes of interest (e.g., cognition) across studies.
In addition to considering how SCD is elicited, it is important to understand if how we record responses (e.g., binary or ordinal scale) affects the association between SCD and outcomes of interest. Although there is evidence that ordinal scales capture important variability in a construct [14, 15], it is also possible that requiring individuals to make a binary judgment regarding the presence of memory difficulties may filter out variability that is not meaningful. However, previous research examining psychological constructs seems to suggest that increasing the number of items leads to improvement in score reliability and that reducing items to two options can reduce measurement precision [15–19]. Given that subjective complaints likely represent a spectrum, Likert scales might be most appropriate to capture variability in SCD.
Endorsement of SCD varies depending on how it is elicited [20]. For example, in a retrospective study by Tandetnik et al. [20], the frequency of SCD was significantly lower when elicited in an age-anchored framework—i.e., when older adults were asked about their cognition in comparison to age-matched peers—than when they were asked about their cognition in general. In that study, age-anchored SCD related more closely to objective memory performance, and in a second study, age-anchored SCD, not retrospective SCD, was linked to amyloid deposition [8, 20]. These findings suggest that age-anchored SCD best captures objective measures of cognitive and brain health in part by normalizing age-related cognitive decline, and/or having less susceptibility to psychosocial factors such as mood or attitudes about aging [21–23].
In all likelihood, the inconsistent association between SCD and objective cognition is not only a product of person and task-specific factors, but the degree to which objective cognitive tests (used as the “gold standard”) are sensitive enough to detect early cognitive dysfunction characteristic of preclinical AD. Efforts are being made to identify measures sensitive to such dysfunction, both by developing novel targeted tasks, as well as combining standard clinical neuropsychological measures to enhance their sensitivity to amyloid positivity and clinical progression in normal controls [24]. In this study, we use two relatively novel computerized tasks, a visual short-term memory binding task and Face-Name associative memory task, as gold standards for the presence of early cognitive dysfunction characteristic of AD. Developed to detect AD-related cognitive change and previously linked to AD biomarkers [25, 26], these tasks can help to hone the utility of SCD for capturing important cognitive variability in clinically normal older adults.
The aim of the current prospective study was to examine the extent to which the association between SCD and objective cognition varies according to how each is measured. Toward this end, the assessment of SCD was manipulated along two dimensions, reference point and response scale, using a within-participant design. The three reference points included: 1) Retrospective (compared to 5 years ago); 2) Age-anchored (compared to others one’s age); and 3) General (no reference point). The two response scales included binary and ordinal (6–point scale). We examined the resulting six SCD measurement frameworks in relation to multiple objective cognitive outcomes of memory including: 1) verbal list learning, a clinical neuropsychological measure frequently used to detect memory decline in MCI and dementia [27, 28] and, 2) cognitive tasks with demonstrated sensitivity to preclinical AD [26, 29]. We had two primary hypotheses: 1) Age-anchored SCD would be endorsed to a lesser extent than SCD as defined against the two other reference points; and 2) Of the six SCD frameworks, age-anchored SCD measured via ordinal scale would relate most strongly to our objective cognitive outcomes. To our knowledge, this is the first prospective manipulation and examination of how task-specific factors influence the link between SCD and objective cognition.
Methods
Participants
A total of 165 cognitively healthy older adults were deemed eligible for this study given our inclusion and exclusion criteria described below. Of these, 32 declined participation, 21 could not be reached/contacted and 2 participants dropped out, leaving a final sample of 110 participants. Participants had a mean age of 72 years (SD=8, range=54 – 90), mean education of 17 years (SD= 2, range=12 – 20), and were 82% White, 12% Black, 3% Asian. Six percent identified themselves as Hispanic. Participants were recruited from the Memory Disorders Clinic (n=7) at Columbia University Medical Center, as well as ongoing studies of cognitive aging with full neuropsychological assessment available including: the Alzheimer’s Disease Research Center, Cognitive Reserve and Reference Ability Neural Network (CR/RANN), and the Testing Olfaction in Primary care to detect Alzheimer’s disease and other Dementias (TOPAD) studies (n=103). As part of the inclusion criteria for this study, participants were required to perform within clinically normal limits (≥ −1.5 SD using demographically adjusted normative data) on standardized assessments of memory, executive function, and language (see supplementary Table S1 for description of tests). SCD was not measured directly as part of the recruitment process, allowing for a spectrum of SCD endorsement. Participants were excluded if they reported or had any past or current neurological conditions such as stroke, traumatic brain injury, brain tumor etc. or major psychiatric disorders noted in their medical records or medical history interview. This study was approved by the Institute Review Board (IRB) at Columbia University as Human Subjects protocol AAAR5197. Participants were consented prior to testing with a full written consent.
Measures
Subjective Cognitive Decline
The SCD questionnaire comprises 20 items, many of which were selected due to their inclusion across several validated SCD questionnaires [30–32]. Items were chosen by a clinical neuropsychologist (S.C.) to span both memory and non-memory abilities. In order to examine how task-specific factors affect the association between SCD and cognition, prospective manipulations of SCD measurement included two rating scales (binary and ordinal) and three reference points. The 20-item SCD questionnaire was administered in its entirety to each participant, in a counterbalanced fashion, using the three separate reference groups defined as: 1) Retrospective (compared to the participant’s own performance 5 years ago); 2) Age-Anchored (compared to others one’s age); and 3) General (no reference point). As highlighted above, for each item, participants provided a binary response (yes/no) followed by a 7-point ordinal scale, regardless of the binary response. These rating scales reflected how much of a ‘problem’ the cognitive complaint was. Specifically, the scale in this study was originally administered using a possible range of 1 (problem) to 7 (no problem scale). However, during analyses and for the purposes of future research, the scale has been revised to 0 (no problem) to 6 (problem) to ensure that the absence of SCD is equal to a score of zero (see Supplementary Table S2 for instructions, items of the questionnaire and recoded items). Examples of the recoded rating scales which were placed in front of participants in an horizontal A4 sheet are included in Supplementary Figure S1–S3. The combination of reference points and response scales produced six separate SCD measurement frameworks including: Retrospective binary, retrospective ordinal, age-anchored binary, age-anchored ordinal, general binary, and general ordinal. Recoded ordinal scales raged from 0–120 while binary scales range from 0–20 with higher scores indicating increased self-reported subjective complaints.
Objective Cognition: Memory
Measures included a clinical neuropsychological list learning test and two more recently developed cognitive tasks shown to be sensitive to cognitive changes in preclinical AD. The Selective Reminding Test (SRT) [27] is the list learning task assessment used to detect episodic memory impairment [33] that is implemented in the clinic and research settings from which the participants were recruited. The SRT is a cued verbal list learning task of 6 trials, with 12 words each. Main outcome measures include Total Recall, ranging from 0 to 72 and Total Delayed Recall, ranging from 0 to 12.
The two cognitive tasks, selected based on evidence that they can detect early cognitive deficits reflective of preclinical AD that are not detected by clinical neuropsychological assessment, were the Short-Term Memory Binding (STMB) and Face-Name Associative Memory Exam (FNAME). The STMB test requires the integration of multi-modal information in short-term memory [25, 34]. Specifically, individuals must integrate two features of a stimulus (shape and color) and hold this representation in their short-term memory [29]. While unaffected by age [35] or non-AD dementia [36], this STMB task has high sensitivity and specificity for preclinical AD when standard clinical neuropsychological measures are within normal limits [29]. The main dependent variable of the STMB task represents total stimuli correctly recognized, ranging from 0–16 with higher scores indicating better performance.
Associative memory was measured with FNAME task [26, 37]. This challenging task requires participants to learn both names and occupations associated with faces. Associative memory is assessed in a learning trial, an immediate memory trial and a delayed memory trial. Performance on the FNAME across names trials has been associated with amyloid burden in healthy older adults, and holds promise as a cognitive indicator of preclinical AD, thus was included as main outcome measure in this study [26]. Thus, our dependent measure included one main score representing associative memory for names (i.e., the sum of name learning immediate and delayed) that ranged from 0–48 with higher scores indicating better performance.
Procedures
All participants completed each of the three versions of the SCD questionnaire, providing both binary (yes/no) ordinal scale (0-6) responses for each item. These measures were counterbalanced across participants (for a total of 6 SCD measurement frameworks) to limit order effects on the responses. Following SCD measurement, participants completed all other cognitive assessments described above, to ensure that experience with the cognitive testing did not directly influence their responses on the SCD interviews.
Statistical Analyses
Statistical analyses were conducted with IBM SPSS v.26 and R v.3.5.2 (R Core Team, 2018). Descriptive statistics were conducted for demographic, SCD and cognitive variables. To test Hypothesis 1, Friedman’s Two-Way Analysis of Variance by Ranks Test for related samples was conducted to examine differences in SCD reference points and scale as a function of reference point as data did not meet assumptions for parametric analysis. Pairwise comparisons with a Bonferroni correction for multiple comparisons were also conducted to examine differences between pairs of SCD reference points. To test Hypothesis 2, Spearman correlations with 1000 sample bootstrapping were conducted to examine associations between ordinal and binary SCD response scales for each reference point and the main outcomes of interest. Confidence intervals from bootstrapping were conducted to be representative of the population in this study (e.g., community recruited individuals) which limits it generalizability to other key population such clinic-based individuals with SCD. A Bonferroni correction for these correlations was set at p=.008. Linear regression models were then conducted to examine the different SCD measurement frameworks in the response scale with strongest associations, after controlling for person-specific factors such as demographic variables and mood, in relation to cognition. Assumptions for regressions were checked examining residuals of each model for lack of significant outliers (> 3 standard deviations on standardized residuals), normality and homoscedasticity.
Results
Subjective Cognitive Decline
Figure 1 depicts the distribution of all SCD scales, and descriptives are included in Table 1. On Likert scales, 4% (n = 4) did not report any General SCD, 5% (n = 5) did not report any retrospective SCD, and 8% (n = 9) did not report any age-anchored SCD. On Binary scales 22% ( n = 24) did not report any General SCD, 26% (n = 29) did not report any retrospective SCD, and 35 (n=32 %) did not report any age-anchored SCD. SCD scales showed good reliability with Cronbach alpha’s ranging from .916 to .932 for ordinal scales and .908 to .913 for binary scales.
Figure 1.
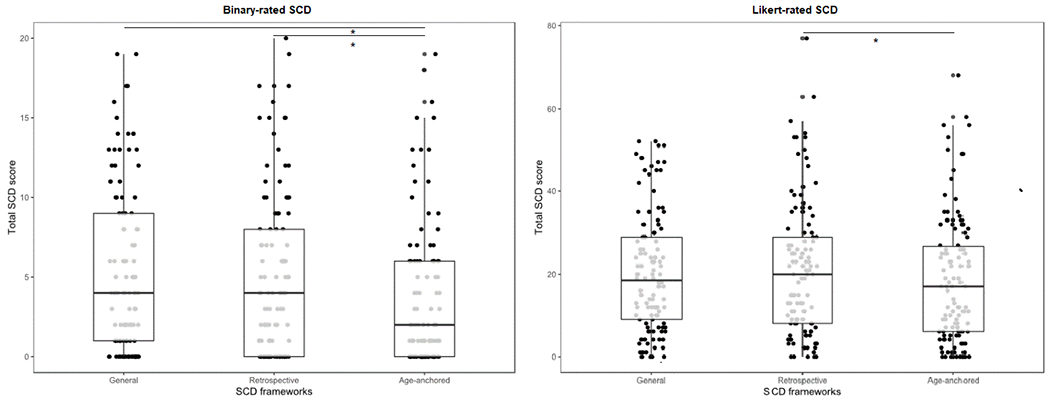
Binary-rated and Ordinal-rated SCD ratings across reference groups.
Boxplot with median and standard error (bars) represented for each SCD reference group. *Significant differences of p value < .05.
Table 1.
Descriptives of SCD endorsement within sample (n=110).
| Descriptives of SCD scales | Mean (SD) | Median(IQR) | Range |
|---|---|---|---|
| Binary-rated SCD | |||
| General | 5.34 (5.21) | 4.00 (8.00) | 0 – 19 |
| Retrospective | 5.14 (5.23) | 4.00 (8.00) | 0 – 20 |
| Age-anchored | 3.51 (4.48) | 2.00 (6.00) | 0 – 20 |
| Ordinal-rated SCD | |||
| General | 20.45 (14.38) | 18.50 (20.75) | 0 – 52 |
| Retrospective | 21.20 (16.15) | 20.00 (21.75) | 0 – 77 |
| Age-anchored | 18.45 (15.36) | 17.00 (68.00) | 0 – 68 |
Friedman’s test revealed significant differences in ordinal-rated SCD across the three reference points (χ2(2)=9.74, p=.008). Specifically, Bonferroni adjusted pairwise comparisons revealed that age-anchored SCD (Mdn=17.00, IQR=21.00) was lower than retrospective SCD (Mdn=20.00, IQR=21.50, p=.009). No significant differences were observed between general SCD (Mdn=18.00, IQR=21.00) versus retrospective (p=.594) or age-anchored SCD (p=.217; See Figure 1). With regard to binary SCD scales, significant differences were also observed in SCD across the three reference points (χ2(2)=31.72, p<.001). Bonferroni adjusted pairwise comparisons revealed that age-anchored SCD was lower (Mdn=2.00, IQR=6.00) than retrospective (Mdn=4.00, IQR=8.00, p<.001) and general (Mdn=4.00, IQR=8.00, p< .001). No significant differences were observed between general and retrospective SCD (p=1.00; see Figure 1). Spearman correlations revealed that all 6 SCD measures were moderately to highly correlated (rho range =.40 - .84, p<.001).
Cognition
Cognitive performance is summarized in Table 2 below.
Table 2.
Raw scores performance on memory measures.
| Mean, standard deviations and range of memory performance | Mean (SD) | Range |
|---|---|---|
| Clinical Memory Assessment | ||
| SRT Total Immediate (raw 0 – 72)* | 52.24 (7.90) | 34 – 70 |
| SRT Total Immediate (percentile) | 64.72 (28.31) | 6.97 – 99.82 |
| SRT Delayed (raw 0 – 12) | 8.45 (2.22) | 4 – 12 |
| SRT Delayed (percentile) | 63.26 (28.98) | 6.68 – 99.11 |
| Cognitive Tasks | ||
| STMB (raw 0 – 16) | 10.89 (1.91) | 6 – 15 |
| FNAME Names (raw 0 – 48) | 18.02 (10.72) | 1 – 45 |
N = 106
Bivariate associations
Binary SCD:
There were no significant associations between cognitive outcomes and binary-rated SCD, in any of the three reference points for the 6 SCD framework analyses (rho-range =−.19 – .05; CI-range=−.40 – .26 ; p-range =.059 – .997). See Table 3 for all bivariate results.
Table 3.
Bivariate associations of memory measures and SCD.
| Spearman Bivariate analyses | FNAME | STMB | SRT Immediate Total Recall | SRT Delayed | ||||
|---|---|---|---|---|---|---|---|---|
| Rho (p-value) | CI | Rho (p-value) | CI | Rho (p-value) | CI | Rho (p-value) | CI | |
| Binary-rated SCD | ||||||||
| Age-anchored | −.14 (.158) | −.32, .06 | −.19 (.059) | −.40, .01 | −.01 (.908) | −.20, .19 | .05 (.591) | −.15, .26 |
| Retrospective | −.19 (.055) | −.37, −.01 | −.14 (.179) | −.32, .06 | −.001 (.997) | −.20, .21 | −.01 (.955) | −.21, .20 |
| General | −.17 (.082) | −.35, .04 | −.03 (.800) | −.22, .16 | −.05 (.638) | −.24, .16 | −.04 (.708) | −.24, .16 |
| Ordinal-rated SCD | ||||||||
| Age-anchored | −.29 (.003)* | −.46, −.09 | −.16 (.101) | −.36, .06 | −.23 (.025) | −.40, −.02 | −.14 (.151) | −.32, .07 |
| Retrospective | −.28 (.004)* | −.45, −.08 | −.11 (.283) | −.30, .09 | −.16 (.107) | −.35, .05 | −.13 (.192) | −.32, .08 |
| General | −.33 (.001)* | −.49, −.14 | −.09 (.397) | −.27, .12 | −.22 (.032) | −.41, −.001 | −.17 (.095) | −.34, .03 |
Note. Significant associations p<.05 are in bold.
Significant after Bonferroni correction p<.008 . CI = Confidence intervals.
Ordinal-rated SCD:
Ordinal rated SCD was associated with SRT Immediate recall in the general (rho =.22, CI = −.41 – −.001, p=.032) and age-anchored framework (rho=.23 CI=−.40 – −.02 p=.025). However, these associations did not survive multiple comparisons adjustment. No significant association were observed with regards to SRT delayed score or the STMB (rho-range =−.17 – −.09; CI-range =−.36 – .12; p-range =.095 – .397). After adjustment for multiple comparisons, all three ordinal-rated SCD scales were associated with performance on the FNAME task (rho-range =−.33 – −.28, CI-range = −.49 – −.08, p-range =.001 – .003) (see Table 3).
Regression analyses
Given the advantage of ordinal over binary SCD response scales observed in bivariate associations, twelve demographically adjusted regression models were conducted to examine the three-ordinal-rated SCD scales (Retrospective, age-anchored, and general) as predictors of the objective cognitive outcome measures. All three models examining SCD as a predictor of associative memory (FNAME) produced similar results (p< .01) with SCD emerging as a significant predictor in each model (p< .05) (see Table 4). Demographics including age and education were also associated with FNAME in all models (p<.05), while gender was associated with FNAME in only one model (e.g., General SCD, p<.05). In contrast, only age-anchored SCD significantly predicted STMB (see Table 5).
Table 4.
Demographically adjusted regression analyses of SCD as a predictor associative memory FNAME.
| Regression models of SCD in relation to FNAME | Model 1 (Age-Anchored) |
Model 2 (Retrospective) |
Model 3 (General) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β | p-value | CI | B | p-value | CI | β | p-value | CI | |
| SCD | −.26 | .018 | −.45, −.06 | −.25 | .012 | −.44, −.06 | −.29 | .003 | −.47, −.10 |
| Age | −.24 | .011 | −.43, −.06 | −.23 | .017 | −.42, −.04 | −.23 | .014 | −.42, −.04 |
| Education | .21 | .027 | .02, .40 | .22 | .023 | .03, .40 | .22 | .019 | .04, .41 |
| Sex (1 – female) |
.16 | .089 | −.02, .34 | .16 | .083 | −.01, .36 | .18 | .047 | .04, .36 |
| Depression | .018 | .853 | −.18, .21 | .003 | .971 | −.16, .17 | .023 | .812 | −.16, .21 |
| Model p-value | .001 | .002 | .001 | ||||||
| R2 | .17 | .17 | .19 | ||||||
| Adjusted R2 | .13 | .13 | .15 | ||||||
| Standard error | 10.02 | 10.08 | 9.92 | ||||||
| Akaike Information Criterion | 508.19 | 504.88 | 506.01 | ||||||
| Schwartz Bayesian Criterion | 524.34 | 520.97 | 522.16 | ||||||
Note. Significance at p<.05 is bolded.
Table 5.
Demographically adjusted regression analyses of SCD as a predictor of visual short term memory binding
| Regression models of SCD as predictor of STMB | Model 1 (Age-Anchored) |
Model 2 (Retrospective) |
Model 3 (General) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β | p-value | CI | β | p-value | CI | β | p-value | CI | |
| SCD | −.22 | .040 | −.45, −.01 | −.11 | .337 | −.33, .10 | −.07 | .515 | −.26, .14 |
| Age | −.11 | .302 | −.32, .10 | −.11 | .303 | −.32, .10 | −.13 | .207 | −.33, .09 |
| Education | .09 | .409 | −.12, .29 | .11 | .302 | −.10, .32 | .11 | .298 | −.10, .32 |
| Sex | .01 | .889 | −.19, .21 | .04 | .685 | −.17, .26 | .04 | .722 | −.16, .25 |
| Depression | −.01 | .931 | −.22, .20 | −.07 | .539 | −.28, .14 | −.07 | .508 | −.28, .14 |
| Model p-value | .212 | .532 | .670 | ||||||
| R2 | .07 | .04 | .03 | ||||||
| Adjusted R2 | .02 | .01 | −.02 | ||||||
| Standard error | 1.89 | 1.91 | 1.92 | ||||||
| Akaike Information Criterion | 135.25 | 137.12 | 139.35 | ||||||
| Schwartz Bayesian Criterion | 150.99 | 152.81 | 155.10 | ||||||
Note. Significance at p<.05 is bolded.
Finally, with regards to performance on a clinical memory assessment (SRT Total Immediate Recall) all three models of SCD significantly predicted SRT Total recall (p< .05; Table 6). However, as shown in Table 7, although all three SCD similarly associated with SRT delayed recall (b = − .17, −.21), only SCD general was significantly associated with it (p<.05).
Table 6.
Demographically adjusted regression analyses of SCD as a predictor of list learning
| Regression models of SCD as predictor of SRT Total Immediate Score | Model 1 (Age-Anchored) |
Model 2 (Retrospective) |
Model 3 (General) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β | p-value | CI | β | p-value | CI | β | p-value | CI | |
| SCD | −.31 | .002 | −.51, −.12 | −.25 | .014 | −.45, −.05 | −.28 | .004 | −.48, −.09 |
| Age | −.13 | .192 | −.33, .07 | −.12 | .273 | −.32, −.09 | −.12 | .265 | −.32, −.08 |
| Education | .14 | .146 | −.05, .33 | .15 | .138 | −.04, .34 | .15 | .116 | −.03, .34 |
| Sex | .01 | .943 | −.002 .002 | .02 | .877 | −.18, .20 | .04 | .666 | −,15, .23 |
| Time^ | .27 | .008 | .07, .46 | .28 | .007 | .07, .48 | .28 | .006 | .08, 48 |
| Depression | .20 | .048 | .002, .39 | .16 | .103 | −.03, .35 | .18 | .073 | .02, .37 |
| Model p-value | .001 | .004 | .002 | ||||||
| R2 | .20 | .18 | .19 | ||||||
| Adjusted R2 | .15 | .12 | .14 | ||||||
| Standard error | 7.30 | 7.45 | 7.35 | ||||||
| Akaike Information Criterion | 424.26 | 424.54 | 425.70 | ||||||
| Schwartz Bayesian Criterion | 442.84 | 443.05 | 444.28 | ||||||
Note. Significance at p<.05 is bolded.
Time between SRT and SCD assessment.
Table 7.
Demographically adjusted regression analyses of SCD as a predictor of list learning delayed recall
| Regression models of SCD as predictor of SRT Delayed Recall Score | Model 1 (Age-Anchored) |
Model 2 (Retrospective) |
Model 3 (General) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β | p-value | CI | β | p-value | CI | β | p-value | CI | |
| SCD | −.17 | .095 | −.37, −.03 | −.19 | .063 | .−.38, .01 | −.21 | .038 | −.40, −.01 |
| Age | −.17 | .108 | −.38, −.04 | −.17 | .144 | −.35, .05 | −.16 | .138 | −.36, .05 |
| Education | .10 | .450 | −.12, .27 | .09 | .395 | −.11, .29 | .08 | .422 | −.17, .27 |
| Sex | .06 | .551 | −.14, .24 | .07 | .487 | −.13, .26 | .07 | .451 | −.12, .27 |
| Time^ | .25 | .016 | .04, .45 | .26 | .014 | .06, .46 | .26 | .013 | .06, .37 |
| Depression | .16 | .102 | −.03, .36 | .16 | .102 | −.03, .35 | .18 | .075 | −.01, .17 |
| Model p-value | .009 | .007 | .005 | ||||||
| R2 | .16 | .17 | .17 | ||||||
| Adjusted R2 | .11 | .11 | .12 | ||||||
| Standard error | 2.12 | 2.11 | 2.10 | ||||||
| Akaike Information Criterion | 162.57 | 160.13 | 160.92 | ||||||
| Schwartz Bayesian Criterion | 181.08 | 178.57 | 179.43 | ||||||
Note. Significance at p<.05 is bolded.
Time between SRT and SCD assessment.
Discussion
This study examined how SCD task specific factors (aspects of SCD measurement) affect its level of endorsement, and its association with sensitive markers of memory functioning among ostensibly cognitively healthy older adults. Specifically, we examined if SCD measurement frameworks including both reference points (i.e., SCD in general, compared to 5 years ago, and compared to others your age), and response scales (i.e., binary versus ordinal) affect level of endorsement, and degree of association with objective memory tasks. Overall, as hypothesized, age-anchored SCD was endorsed less frequently than general or retrospective SCD. Regarding response scale (i.e., binary versus ordinal), correlational analyses showed that ordinal-rated SCD was associated with cognitive outcomes to a relatively higher extent than the binary-rated SCD. Finally, regression analyses revealed that age-anchored SCD, measured using an ordinal rating scale, mapped most consistently onto objective cognitive measures which were carefully selected to be sensitive to memory deficits that may emerge early in the context of AD.
With respect to response scale, ordinal ratings appeared preferable to binary ratings. Indeed, ordinal scales may capture a more comprehensive and fine-grained picture of the construct of interest, in this case, SCD, than is possible with a binary rating. There is ample research from educational and psychological disciplines examining differences and overall utility of various response scales. While some research has found no meaningful differences across binary versus ordinal scales [38], others have found ordinal scales to produce more stable results and reduce measurement error [14, 15]. Indeed, multiple studies have found that increasing response options from two to seven can increase reliability, validity and discriminability [14, 15]. To some extent, the utility of ordinal scales may reflect differences in person specific factors such as individuals’ response biases, as individuals likely vary in their threshold for shifting from a “No” response to a “Yes” response. That is, two individuals may endorse a score of 4 on an ordinal scale, but differ in their binary response. Results from the current study support the use of ordinal scales in quantifying SCD in order to capture variability in objective memory among cognitively normal older adults.
Another SCD task specific factor that was examined in this study was the manipulation of reference points. Results from this study showed that age-anchored SCD was endorsed the least, and was most frequently associated with the memory outcomes. Given the high prevalence of cognitive complaints in older adults [39, 40] it is important that we distinguish between complaints that may be age-related versus those which may reflect incipient disease. Increased endorsement of retrospective and general SCD compared to age-anchored suggests that by providing an age-anchor, individuals focus on those complaints that go beyond those they feel they can attribute to age. Indeed, in the most recent SCD working group, SCD compared to others one’s age was identified as an SCD plus criterion – a criterion considered to increase the likelihood of preclinical AD [12]. It should be acknowledged that other factors may influence the way in which people adjust their ratings across SCD reference groups; for example, age-anchored could be least endorsed as a reaction to social threat, such that individuals rate their abilities in a more positive light than others their age [41, 42]. If this were the case, however, one would expect this response pattern to obscure the association between SCD and cognition.
A secondary issue examined in this study was the extent to which SCD mapped on to cognitive functioning depending on the instrument selected as the main outcome. Efforts are being made to identify tests which are most sensitive to the earliest stages of AD, and recent clinical trials have suggested that a composite score of several clinical neuropsychological measures is useful in tracking progression among amyloid positive normal controls[24]. In the current study, we assessed cognitive functions not typically measured as part of a clinical neuropsychological assessment, including visual short term memory binding and face-name associative memory, both shown to be sensitive to AD biomarkers [25, 26]. Among these cognitive tasks, the current study found divergent results; while the FNAME was associated with all SCD frameworks, the STMB was associated with age-anchored SCD only, potentially reflecting inherent differences within these tasks. For example, the STMB seems to be both sensitive and specific to early changes in patients with AD and not with key demographic factors such as age or education [25, 36]. On the other hand, the FNAME has been shown to be associated with age [43]. The current study replicated these differential associations, and also suggested that FNAME was associated with education as well. It may therefore be that FNAME captures elements of age-related cognitive difficulties or educational background that reveal themselves in each of the three SCD frameworks. However, the associations between SCD and FNAME remained after adjusting for these demographic variables, leaving the dissociation between STMB and FNAME an open question. It is possible that the FNAME captures elements of subjective cognition that reflect a wider range of factors (e.g., biological and/or social) than do the other cognitive tasks administered in the current study. Longitudinal data tracking the evolution of performance on cognitive outcomes over time will help to clarify the relevance of the differential associations between these outcomes and different types of SCD at baseline.
Finally, with respect to a traditional clinical assessment tool, demographically adjusted models showed that SRT Total Immediate Recall score was associated with all SCD assessments. With regards to delayed recall, although all SCD reference points had similar effect sizes only general SCD was significantly associated with delayed recall. These results were in line with those observed with the associative memory test. These results showed that at least in this sample SCD was able to capture early cognitive dysfunction within traditional cognitive assessments.
Taken together, these findings support the idea that age-anchoring SCD assessment and asking older adults to rate their experience on an ordinal-scale optimizes its association with important measures of cognition including associative verbal memory, short-term memory binding of features, and list learning. The relative value of age-anchored SCD, consistent with findings from previous imaging and cognitive studies [8, 20], is in line with the idea that a certain degree of cognitive change such as memory decline is expected and experienced with typical aging, and underscores the importance of refining SCD assessment. Age-anchored SCD can be a clinically meaningful screener given its sensitivity to a range of early memory difficulties previously shown to be associated with biomarkers of AD in cognitively normal individuals. In contrast to administering the measures themselves which requires at least 30 minutes of a computer-based administration, SCD can be assessed quickly and easily, by phone or mail if necessary, and requires no training or specific administration procedures. The relative clinical utility, or unique value, of SCD versus these objective tasks as indicators of preclinical AD remains to be determined and is the focus of ongoing work
This study has several potential limitations that should be considered when interpreting results. First, although age-anchored SCD was endorsed to a lesser degree than retrospective or general SCD, the total SCD scores across the reference point frameworks were relatively similar. This similarity might have arisen specifically as a function of the within-person design of the study in which each participant completed all three SCD frameworks consecutively (in a counterbalanced order across participants). Such a design, while resistant to differences across individuals, may result in a “blending together” of responses or response style across the 60 SCD items rated in close proximity to one another. A between-subjects design may have detected a larger difference between age-anchored SCD versus general or retrospective SCD. Spacing the SCD ratings apart might also have detected a larger difference; however, such an approach would require the introduction of other assessments in the interim time which could potentially influence SCD ratings, raising a new set of challenges. Further, although reference point was counterbalanced, SCD response scale (e.g., binary vs ordinal) was not. Participants always endorse binary prior to ordinal and thus it is not clear if order effects might have impacted the way that individuals responded to these scales. Participants from this study were included if they performed within normal limits on neuropsychological measures irrespective of baseline SCD. This could have reduced the power of our study as many individuals did not experience any SCD, and the current study was not powered to formally examine differences in SCD as a function of recruitment source. Indeed, there were only 7 participants recruited from the clinic; previous work has demonstrated that individuals report higher SCD in clinic-based samples than in community based samples [44]. The low number of clinic-based participants in the current sample reflects the fact that most individuals coming into the Aging and Dementia clinic with cognitive complaints were found to have some level of cognitive impairment on testing, and/or to have other documented neurologic or psychiatric disease. Future studies should directly examine the potentially moderating role of recruitment source on the association between SCD measurement frameworks and cognitive outcomes.
To conclude, this study highlights the importance of considering both SCD and cognitive measures when determining the utility of SCD as a marker of preclinical AD. This study showed that ordinal-rated, age-anchored SCD most closely approximates objective memory functioning above and beyond several person-specific factors such as demographics and mood (e.g., depression). Further work is needed to examine additional task-specific factors such as whether measuring concern about memory difficulties strengthens the link between SCD and objective cognition, as well as evaluating the extent to which other person-specific factors not examined in this study (e.g., race/ethnicity, personality, attitudes about aging, or metacognition may moderate the association between SCD and objective markers of AD observed in this study. Simultaneous consideration of both task and person-specific factors is critical for optimal modeling of SCD, and empirically-based development of SCD assessments for detecting preclinical AD.
Supplementary Material
Supplementary Figure S1. Sheet 1 with rating scales for general.
Supplementary Figure S2. Sheet 2 with rating scales for SCD retrospective.
Supplementary Figure S3. Sheet 3 with rating scales for SCD age-anchored.
Acknowledgments
Funding: This work was supported by the National Institutes of Health (1R01AG054525-01A1) and the Mortimer B. Zuckerman Family Foundation.
Footnotes
Financial disclosures: All authors declare no financial disclosures relevant to this study.
References
- [1].Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr., Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Negash S, Wilson RS, Leurgans SE, Wolk DA, Schneider JA, Buchman AS, Bennett DA, Arnold SE (2013) Resilient brain aging: characterization of discordance between Alzheimer’s disease pathology and cognition. Curr Alzheimer Res 10, 844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Geerlings MI, Jonker C, Bouter LM, Ader HJ, Schmand B (1999) Association between memory complaints and incident Alzheimer’s disease in elderly people with normal baseline cognition. Am J Psychiatry 156, 531–537. [DOI] [PubMed] [Google Scholar]
- [5].Reisberg B, Prichep L, Mosconi L, John ER, Glodzik-Sobanska L, Boksay I, Monteiro I, Torossian C, Vedvyas A, Ashraf N, Jamil IA, de Leon MJ (2008) The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer’s disease. Alzheimers Dement 4, S98–s108. [DOI] [PubMed] [Google Scholar]
- [6].Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, Maye JE, Gidicsin C, Pepin LC, Sperling RA, Johnson KA, Rentz DM (2012) Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia 50, 2880–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Amariglio RE, Mormino EC, Pietras AC, Marshall GA, Vannini P, Johnson KA, Sperling RA, Rentz DM (2015) Subjective cognitive concerns, amyloid-beta, and neurodegeneration in clinically normal elderly. Neurology 85, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ (2012) Subjective Cognition and Amyloid Deposition Imaging: A Pittsburgh Compound B Positron Emission Tomography Study in Normal Elderly Individuals. Arch Neurol 69, 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wolfsgruber S, Jessen F, Koppara A, Kleineidam L, Schmidtke K, Frolich L, Kurz A, Schulz S, Hampel H, Heuser I, Peters O, Reischies FM, Jahn H, Luckhaus C, Hull M, Gertz HJ, Schroder J, Pantel J, Rienhoff O, Ruther E, Henn F, Wiltfang J, Maier W, Kornhuber J, Wagner M (2015) Subjective cognitive decline is related to CSF biomarkers of AD in patients with MCI. Neurology 84, 1261–1268. [DOI] [PubMed] [Google Scholar]
- [10].Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B (2014) Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand 130, 439–451. [DOI] [PubMed] [Google Scholar]
- [11].Kryscio RJ, Abner EL, Cooper GE, Fardo DW, Jicha GA, Nelson PT, Smith CD, Van Eldik LJ, Wan L, Schmitt FA (2014) Self-reported memory complaints. Implications from a longitudinal cohort with autopsies Neurology 83, 1359–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, Dubois B, Dufouil C, Ellis KA, van der Flier WM, Glodzik L, van Harten AC, de Leon MJ, McHugh P, Mielke MM, Molinuevo JL, Mosconi L, Osorio RS, Perrotin A, Petersen RC, Rabin LA, Rami L, Reisberg B, Rentz DM, Sachdev PS, de la Sayette V, Saykin AJ, Scheltens P, Shulman MB, Slavin MJ, Sperling RA, Stewart R, Uspenskaya O, Vellas B, Visser PJ, Wagner M, Subjective Cognitive Decline Initiative Working G (2014) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 10, 844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rabin LA, Smart CM, Crane PK, Amariglio RE, Berman LM, Boada M, Buckley RF, Chételat G, Dubois B, Ellis KA, Gifford KA, Jefferson AL, Jessen F, Katz MJ, Lipton RB, Luck T, Maruff P, Mielke MM, Molinuevo JL, Naeem F, Perrotin A, Petersen RC, Rami L, Reisberg B, Rentz DM, Riedel-Heller SG, Risacher SL, Rodriguez O, Sachdev PS, Saykin AJ, Slavin MJ, Snitz BE, Sperling RA, Tandetnik C, van der Flier WM, Wagner M, Wolfsgruber S, the Alzheimer’s Disease Neuroimaging I, Sikkes SAM, the Subjective Cognitive Decline Initiative Working G (2015) Subjective Cognitive Decline in Older Adults: An Overview of Self-Report Measures Used Across 19 International Research Studies. J Alzheimers Dis 48, S63–S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Preston CC, Colman AM (2000) Optimal number of response categories in rating scales: reliability, validity, discriminating power, and respondent preferences. Acta Psychol (Amst) 104, 1–15. [DOI] [PubMed] [Google Scholar]
- [15].Simms LJ, Zelazny K, Williams TF, Bernstein L (2019) Does the number of response options matter? Psychometric perspectives using personality questionnaire data. Psychol Assess 31, 557–566. [DOI] [PubMed] [Google Scholar]
- [16].Flynn D, van Schaik P, van Wersch A (2004) A Comparison of Multi-Item Likert and Visual Analogue Scales for the Assessment of Transactionally Defined Coping Function. Eur J Psychol Assess 20, 49–58. [Google Scholar]
- [17].Flamer S (1983) Assessment of the Multitrait-Multimethod Matrix Validity of Likert Scales Via Confirmatory Factor Analysis. Multivariate Behav Res 18, 275–306. [DOI] [PubMed] [Google Scholar]
- [18].Hilbert S, Küchenhoff H, Sarubin N, Nakagawa TT, Bühner M (2016) The influence of the response format in a personality questionnaire: An analysis of a dichotomous, a Likert-type, and a visual analogue scale. TPM-Test, Psychom Methodol in Appl Psychol 23, 3–24. [Google Scholar]
- [19].Weng L-J (2004) Impact of the Number of Response Categories and Anchor Labels on Coefficient Alpha and Test-Retest Reliability. Educational and Psychological Measurement 64, 956–972. [Google Scholar]
- [20].Tandetnik C, Farrell MT, Cary MS, Cines S, Emrani S, Karlawish J, Cosentino S (2015) Ascertaining Subjective Cognitive Decline: A Comparison of Approaches and Evidence for Using an Age-Anchored Reference Group. J Alzheimers Dis 48 Suppl 1, S43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hill NL, Mogle J, Wion R, Munoz E, DePasquale N, Yevchak AM, Parisi JM (2016) Subjective Cognitive Impairment and Affective Symptoms: A Systematic Review. Gerontologist 56, e109–e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Koller OM, Hill NL, Mogle J, Bhang I (2019) Relationships Between Subjective Cognitive Impairment and Personality Traits: A Systematic Review. J Gerontol Nurs 45, 27–34. [DOI] [PubMed] [Google Scholar]
- [23].Bolla KI, Lindgren KN, Bonaccorsy C, Bleecker ML (1991) Memory complaints in older adults. Fact or fiction? Arch Neurol 48, 61–64. [DOI] [PubMed] [Google Scholar]
- [24].Donohue MC, Sperling RA, Salmon DP, Rentz DM, Raman R, Thomas RG, Weiner M, Aisen PS (2014) The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol 71, 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Parra MA, Abrahams S, Fabi K, Logie R, Luzzi S, Sala SD (2009) Short-term memory binding deficits in Alzheimer’s disease. Brain 132, 1057–1066. [DOI] [PubMed] [Google Scholar]
- [26].Rentz DM, Amariglio RE, Becker JA, Frey M, Olson LE, Frishe K, Carmasin J, Maye JE, Johnson KA, Sperling RA (2011) Face-name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia 49, 2776–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Buschke H (1984) Cued recall in Amnesia. Journal of Clinical Neuropsychology 6, 433–440. [DOI] [PubMed] [Google Scholar]
- [28].Hannay HJ, Levin HS (1985) Selective reminding test: an examination of the equivalence of four forms. J Clin Exp Neuropsychol 7, 251–263. [DOI] [PubMed] [Google Scholar]
- [29].Parra MA, Abrahams S, Logie RH, Méndez LG, Lopera F, Della Sala S (2010) Visual short-term memory binding deficits in familial Alzheimer’s disease. Brain 133, 2702–2713. [DOI] [PubMed] [Google Scholar]
- [30].Gilewski M, Zelinski E, Schaie K (1990) The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychol aging 5, 482–490. [DOI] [PubMed] [Google Scholar]
- [31].Rami L, Mollica M, García-Sánchez C, Saldaña J, Sánchez-Saudinós M, Sala I, Valls-Pedret C, Castellvi M, Olives J, Molinuevo J (2014) The Subjective Cognitive Decline Questionnaire (SCD-Q): A Validation Study. J Alzheimers Dis 41, 453–466. [DOI] [PubMed] [Google Scholar]
- [32].Farias S, Mungas D, Reed B, Cahn-Weiner D, Jagust W, Baynes K, Decarli C (2008) The Measurement of Everyday Cognition (ECog): Scale Development and Psychometric Properties. Neuropsychology 22, 531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Parra MA, Sala SD, Abrahams S, Logie RH, Méndez LG, Lopera F (2011) Specific deficit of colour–colour short-term memory binding in sporadic and familial Alzheimer’s disease. Neuropsychologia 49, 1943–1952. [DOI] [PubMed] [Google Scholar]
- [35].Parra MA, Abrahams S, Logie RH, Sala SD (2009) Age and binding within-dimension features in visual short-term memory. Neurosci Lett 449, 1–5. [DOI] [PubMed] [Google Scholar]
- [36].Della Sala S, Parra MA, Fabi K, Luzzi S, Abrahams S (2012) Short-term memory binding is impaired in AD but not in non-AD dementias. Neuropsychologia 50, 833–840. [DOI] [PubMed] [Google Scholar]
- [37].Sperling RA, Bates JF, Cocchiarella AJ, Schacter DL, Rosen BR, Albert MS (2001) Encoding novel face-name associations: A functional MRI study. Hum Brain Mapp 14, 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Matell MS, Jacoby J (1971) Is There an Optimal Number of Alternatives for Likert Scale Items? Study I: Reliability and Validity. Educat Psychol Meas 31, 657–674. [Google Scholar]
- [39].Jonker C, Geerlings MI, Schmand B (2000) Are memory complaints predictive for dementia? A review of clinical and population-based studies. In J Geriatr. Psychiatry 15, 983–991. [DOI] [PubMed] [Google Scholar]
- [40].Begum A, Dewey M, Hassiotis A, Prince M, Wessely S, Stewart R (2014) Subjective cognitive complaints across the adult life span: a 14-year analysis of trends and associations using the 1993, 2000 and 2007 English Psychiatric Morbidity Surveys. Psychol Med 44, 1977–1987. [DOI] [PubMed] [Google Scholar]
- [41].Alicke MD (2000) Evaluating Social Comparison Targets In Handbook of Social Comparison: Theory and Research, Suls J, Wheeler L, eds. Springer US, Boston, MA, pp. 271–293. [Google Scholar]
- [42].Fastame MC, Penna MP, Rossetti ES, Agus M (2012) Perceived Well-Being and Metacognitive Efficiency in Life Course: A Developmental Perspective. Res Aging 35, 736–749. [Google Scholar]
- [43].Amariglio RE, Frishe K, Olson LE, Wadsworth LP, Lorius N, Sperling RA, Rentz DM (2012) Validation of the Face Name Associative Memory Exam in cognitively normal older individuals. J Clin Exp Neuropsychol 34, 580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Perrotin A, La Joie R, de La Sayette V, Barré L, Mézenge F, Mutlu J, Guilloteau D, Egret S, Eustache F, Chételat G (2017) Subjective cognitive decline in cognitively normal elders from the community or from a memory clinic: Differential affective and imaging correlates. Alzheimers Dement 13, 550–560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Sheet 1 with rating scales for general.
Supplementary Figure S2. Sheet 2 with rating scales for SCD retrospective.
Supplementary Figure S3. Sheet 3 with rating scales for SCD age-anchored.


