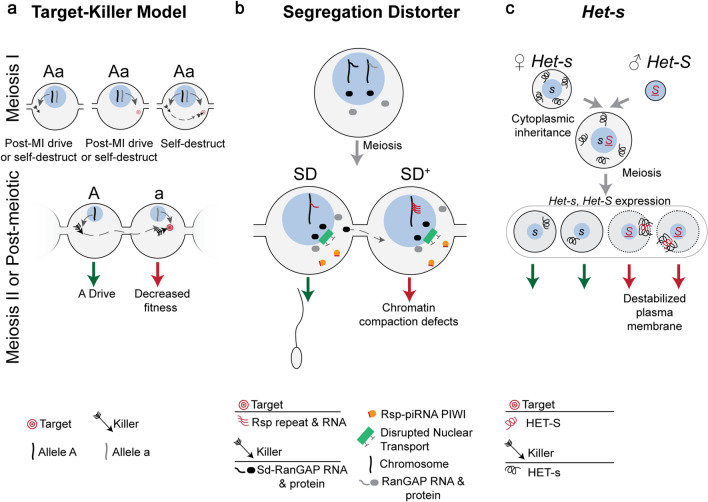
Fig. 3.
Symmetric Meiotic Drive—Target–Killer. This figure depicts the theoretical importance of intracellular versus intercellular regulation on target–killer meiotic drive systems (a), and the mechanisms underlying two target–killer meiotic drivers (b and c). a The killer protein (black arrow, encoded on the “A” haplotype) targets, directly or indirectly, the target (red bullseye, encoded on the “a” haplotype). During meiosis I (MI), expression of only the killer before or during MI can result in post-MI drive, if the target is a trans-acting factor expressed during or after meiosis II (MII). If the target is a cis-acting sequence, expression of the killer before or during MI could cause the driver to self-destruct. If only a trans-acting target is expressed during MI, post-MI drive occurs if the killer is expressed during or after MII, assuming the target acts intracellularly. However, if the target is partitioned into all MII cells and is therefore intercellular, then expression of the killer will result in the system self-destructing. If a trans-acting killer is expressed during MI and a cis-acting or trans-acting target are accessible, then the system will self-destruction. The target and killer are present in different cells and on different haplotypes in order to not self-destruct, as would be the case if both were expressed in meiotic cells. Expression of the target or killer following homologous chromosome separation in MII prevents self-destruction and results in meiotic drive (green and red arrows). Post-meiosis II expression of the killer necessitates sharing (dashed gray arrow) through cytoplasmic bridges, the target should not be shared in order to prevent self-destruction. b Drosophila melanogaster Segregation Distorter (SD) encodes the killer Sd-RanGAP. One model proposes that Sd-RanGAP mislocalization disrupts piRNA-based silencing (orange) of the target, the high copy number Rsp (red lines) resulting in chromatin compaction defects and SD drive. c The female strain of P. anserina encodes the killer allele, Het-s, and HET-s protein (black curly line) is therefore present upon gamete fusion as the cytoplasm (gray surrounding blue nucleus) is maternally inherited. The male strain’s Het-S allele and female Het-s are expressed following meiosis where they complex in Het-S spores and integrate and destabilize the plasma membrane (red arrow), resulting in Het-s drive (green arrow). Cells are contained within an ascus (large gray oval)