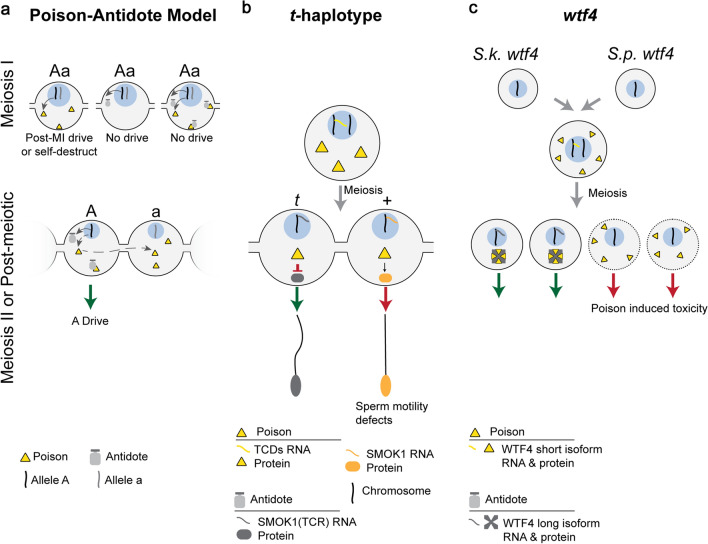
Fig. 4.
Symmetric Meiotic Drive—Poison-Antidote. This figure depicts the theoretical importance of intracellular versus intercellular regulation on poison–antidote meiotic drive systems (a), and the mechanisms underlying two poison-antidote meiotic drivers (b and c). a The poison protein (yellow triangle) and antidote (gray shape) are encoded by the same haplotype (“A”) in close genetic proximity. The antidote acts intracellularly (“A” haplotype) to spare driving chromosomes from the effects of the intercellular poison. Expression of the poison before or during MI can result in post-MI drive, if the poison acts specifically in MII or post-meiosis. However, if the poison acts on a biological pathway present before or during MI, expression before or during MI leads to self-destruction. Expression of the antidote before or during MI would result in it being partitioned into and consequently sparing all daughter cells, preventing drive. Expression of both the poison and antidote before homologous chromosomes separate would result in the antidote neutralizing the poison and preventing drive. Meiosis II or later expression of the poison requires that the poison is shared through cytoplasmic bridges (dashed gray arrow) to poison the other cell containing the “a” haplotype, resulting in A drive (green arrow). b In the mouse t-haplotype, the poisons, TCDs (yellow triangle), are expressed before meiosis and disrupt the post-meiotically expressed SMOK1 (orange oval) resulting in flagellar dysregulation (red arrow). The antidote, SMOK1(TCR) (gray oval), is a dominant negative variant of SMOK1 and is insensitive to TCD induced dysregulation, resulting in t-haplotype drive (green arrow). c In Schizosaccharomyces, the wtf4 gene encoded both the pre-meiotically expressed poison (yellow triangle) and the post-meiotically expressed antidote (gray shape). These represent distinct isoforms of wtf4. The antidote sequesters poison into distinct subcellular regions preventing its toxicity and results in drive (green arrow)