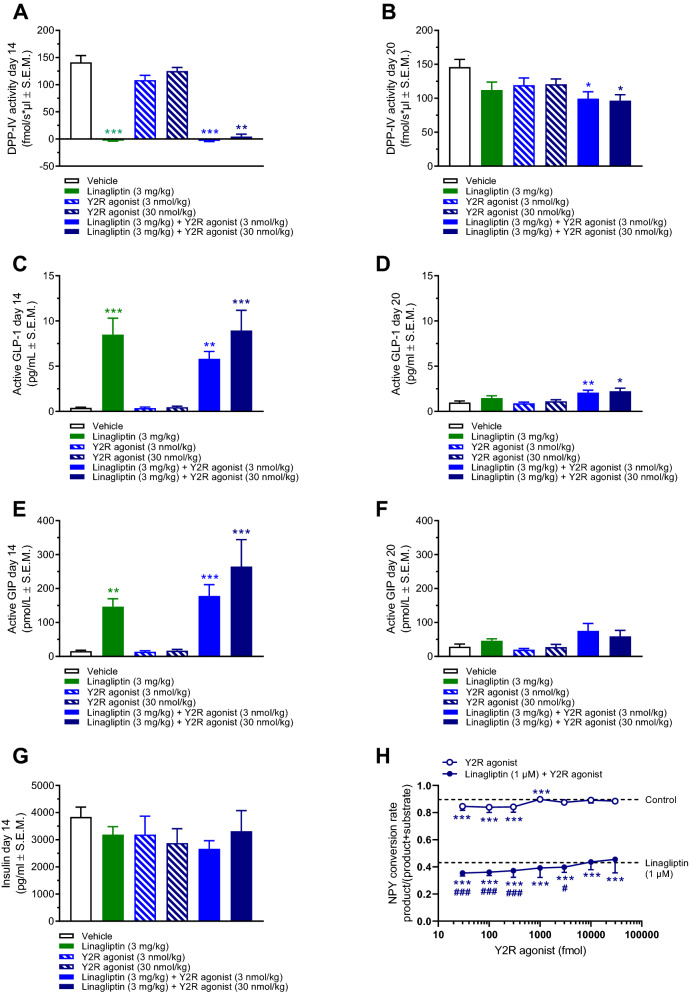
Figure 6.
Linagliptin-induced DPP-IV inhibition is unaffected by Y2R agonist co-administration. Linagliptin (3 mg/kg, SC) and long-acting Y2 receptor agonist (PYY analogue, 3 or 3 nmol/kg, SC) was administered once daily for 14 days followed by 6 days of wash-out (no treatment) before study termination. (A) Plasma DPP-IV activity measured on treatment day 14 (4 h post-dosing). (B) Terminal plasma DPP-IV activity measured on study day 20. (C) Plasma active GLP-1 levels measured on treatment day 14 (4 h post-dosing). (D) Terminal plasma active GLP-1 levels measured on study day 20. (E) Plasma active GIP levels measured on treatment day 14 (4 h post-dosing). (F) Terminal plasma active GIP levels measured on study day 20. *p < 0.05, **p < 0.01, ***p < 0.001 vs. vehicle control (Kruskal–Wallis test). (G) Plasma insulin levels measured on treatment day 14 (4 h post-dosing). (H) NPY conversion rate in human EDTA-plasma added linagliptin (1 µM), Y2 receptor agonist (30–30,000 fmol) or linagliptin (1 µM) + Y2R agonist (30–30,000 fmol). ***p < 0.001 vs. control, #p < 0.05, ###p < 0.001 vs. linagliptin alone (Welch’s t-test).