Figure 2.
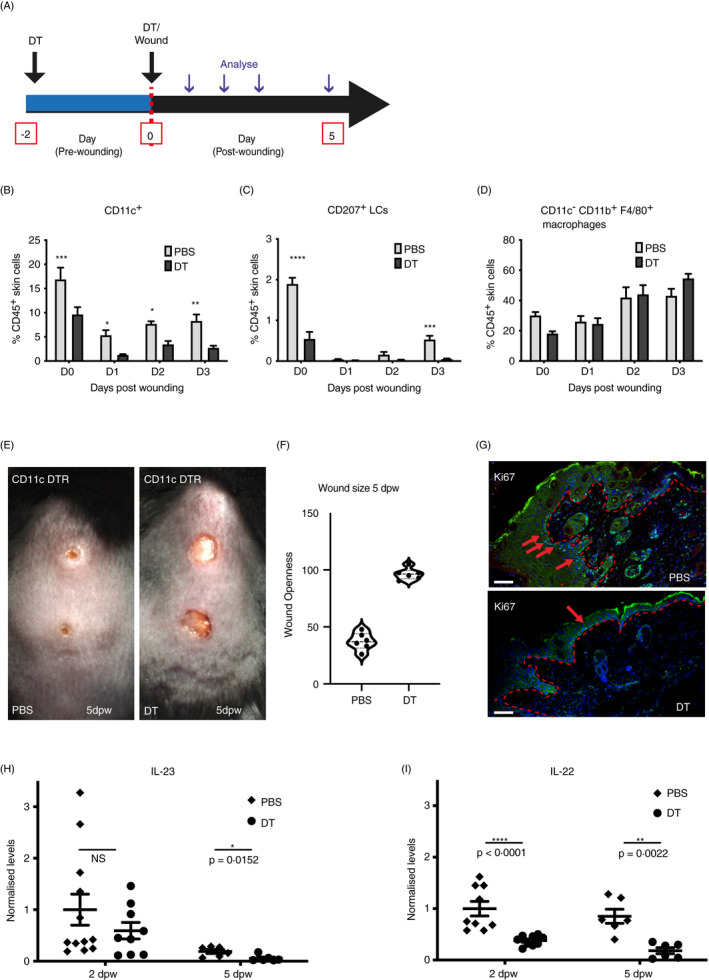
Ablation of CD11c+ cells delays wound closure and IL‐23/IL‐22 production in wounds. (A) Schematic showing the experimental protocol. CD11c‐DTR mice were injected with DT or PBS, as a control, 2 days prior to and on the day of wounding. (B‐D) Wounds were immunophenotyped by flow cytometry. Summary bar graphs show the mean frequency ± SD of all (B) CD11c+ cells (C), CD11bintCD207+ LCs and (D) CD11cnegCD11b+F4/80+ macrophages among total CD45+ cells, in unwounded (D0) and 1, 2 or 3 dpw in both DT‐treated and PBS‐treated CD11c‐DTR mice. Data are from three biological replicates. (E) Representative images of wounded PBS‐treated and DT‐treated CD11c‐DTR mice 5 dpw. (F) Wounds were photographed, and size was calculated as a percentage of the wound size on the day of wounding. Graphs show individual mice with lines at the mean. (G) Sections of wounds 5 dpw from PBS‐ and DT‐treated mice stained with an antibody to Ki67 (green) and costained with DAPI (blue). Red arrows indicate representative positive staining, and red asterisks mark epidermis adjacent to wound sites. Scale bars equal 100 microns. (H and I) qPCR quantification of IL‐23 and IL‐22 in total wound skin from PBS‐ and DT‐treated CD11c‐DTR mice analysed 2 and 5 dpw. Graphs show individual samples, with lines at mean ± SD, n = 9–12. Each time‐point was compared with unwounded skin (B‐D) or PBS‐treated control at 2 or 5 dpw (H and I) using the pairwise comparison t‐test with the Welsh correction. *P ≤ 0·05; **P ≤ 0·01; ***P ≤ 0·001; and ****P ≤ 0·0001; NS, not significant.
