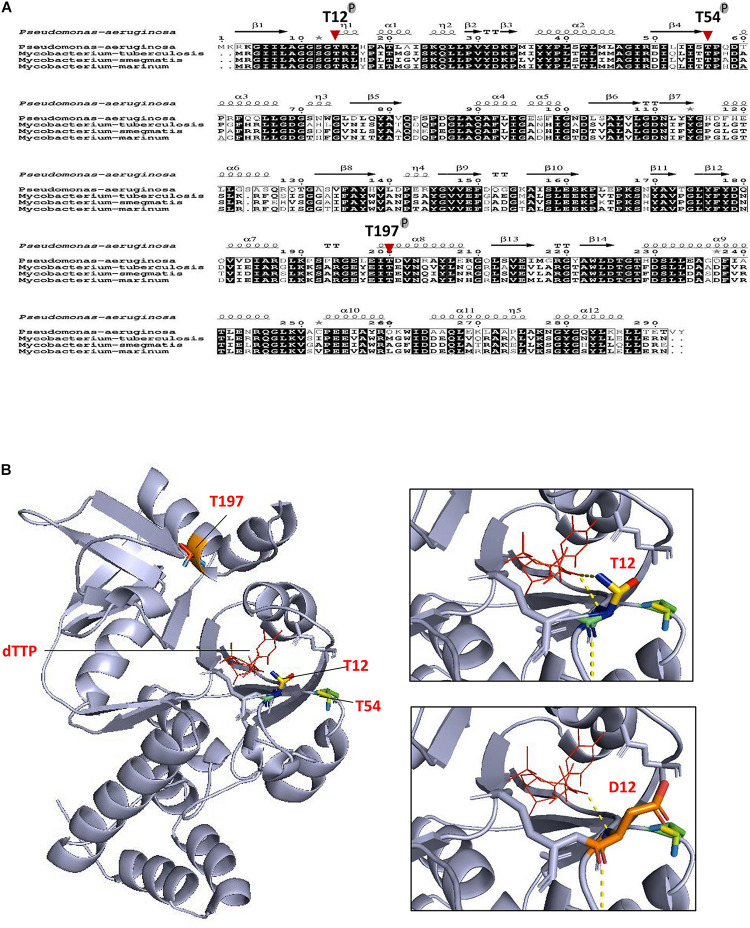
FIGURE 3.
Conservation of phosphoacceptors in RmlA orthologs. (A) Multiple sequence alignment of RmlA sequences from Pseudomonas aeruginosa to mycobacterial species. The alignment was performed using ClustalW and Espript. Residues conserved in all species are shown in black boxes. The three phosphorylation sites of RmlA are indicated. Protein secondary element assignments are represented above the sequences. Numbering of amino acids corresponds to the RmlA protein from P. aeruginosa. (B) Localization of T12, T54, T197 phospho-sites in the three-dimensional structure of RmlA. Overall view (left panel) showing the RmlA monomer structure in ribbon representation with the α-helix and the β-sheet. Close-up view (right panel) indicates side chains of residues delineating the active site hydrophobic tunnel.