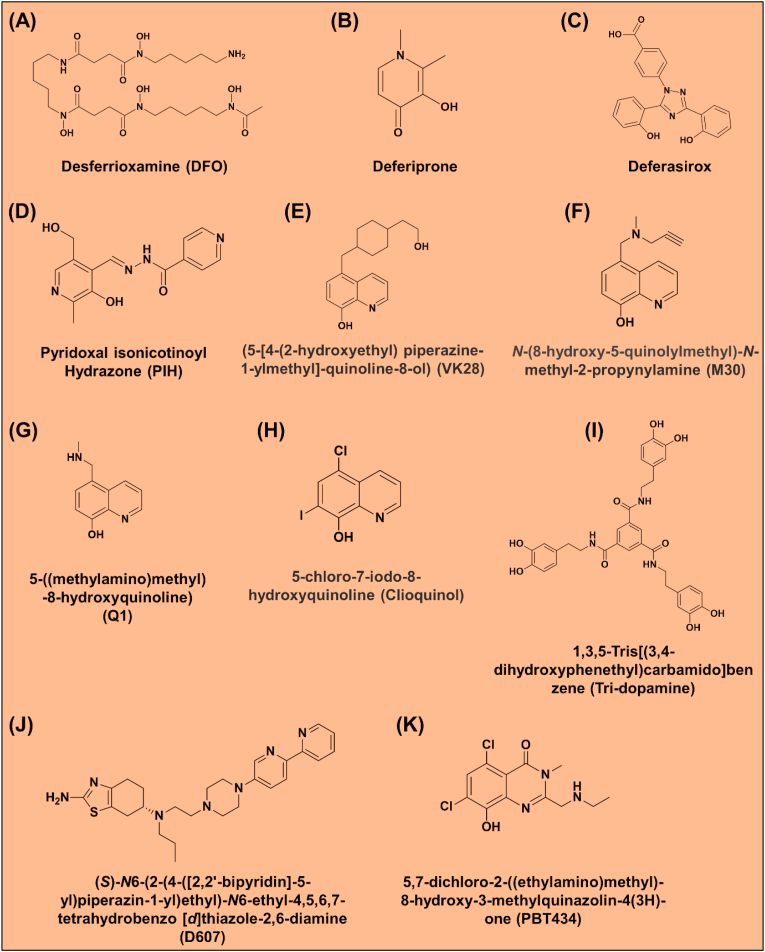
Fig. 9.
Line drawings of the molecular structures of: (A) Desferrioxamine (DFO); (B) Deferiprone; (C) Deferasirox; (D) pyridoxal isonicotinoyl hydrazone (PIH); (E) (5-[4-(2-hydroxyethyl) piperazine-1-ylmethyl]-quinoline-8-ol) (VK28); (F) N-(8-hydroxy-5-quinolylmethyl)-N-methyl-2-propynylamine M30; (G) 5-((methylamino)methyl)-8-hydroxyquinoline) (Q1); (H) 5-chloro-7-iodo-8-hydroxyquinoline (clioquinol); (I)tris-dopamine ligand 1,3,5-tris[(3,4-dihydroxyphenethyl)carbamido]benzene; (J) (S)–N6-(2-(4-([2,2′-bipyridin]-5-yl)piperazin-1-yl)ethyl)-N6-ethyl-4,5,6,7-tetrahydrobenzo [d]thiazole-2,6-diamine (D607); and (K) 5,7-dichloro-2-((ethylamino)methyl)-8-hydroxy-3-methylquinazolin-4(3H)-one (PBT434).