Abstract
Alpinia eremochlamys K. Schum, Etlingera flexuosa A.D. Poulsen, and Etlingera acanthoides A.D. Poulsen are endemic Zingiberaceae plants from Central Sulawesi, Indonesia. This study is the first report on screening the potential antiviral activity of ethanol extracts of the leaves, pseudostems, and rhizomes parts on HIV-infected MT-4 cells and identifying chemical constituents by GC-MS. The plants were extracted by the maceration method using 96% ethanol as a solvent. The antiviral activity was measured using Viral-ToxGlo colorimetric method and using the extracts at concentrations ranging from 7.8 to 1000 μg/mL. GC-MS was used to identify the secondary metabolites of potential extracts. The results showed that ethanol extract of E. acanthoides rhizome was the most potent antiviral activity (IC50 of 1.74 ± 2.46 μg/mL) and less toxic on lymphocyte (MT-4) cells (CC50 of 204.90 ± 106.35 μg/mL), affording the highest value of selectivity index (SI) of 117.76. A. eremochlamys rhizomes also showed promising antiviral activity with IC50 of 64.18 ± 2.58 μg/mL and no toxicity on MT-4 cells affording a high SI value 19.05. Preliminary GC-MS identification showed the presence of terpenoids and fatty acids as major compounds. Zerumbone, ar-turmerone, caryophyllene, and caryophyllene oxide were also detected. Chemical constituents identified by GC-MS might be responsible for the antiviral activity of extracts, suggesting further isolation and antiviral testing of the purified compounds.
Keywords: Zingiberaceae, Endemic, HIV-1, MT-4, GC-MS
Zingiberaceae, endemic, HIV-1, MT-4, GC-MS.
1. Introduction
Human immunodeficiency virus type-1 (HIV-1) is a member of the retrovirus family, and is responsible for causing acquired immunodeficiency syndrome (AIDS). As of 2019, about 38.0 million people have been reported to be living with HIV, and 690,000 people have died due to AIDS-related illnesses [1]. Currently, the management of HIV infection involves antiretroviral drugs with a complex dose regimen because these drugs have a mechanism of action that suppresses viral replication. However, adverse effects, including drug resistance, do occur with prolonged usage of these drugs. Therefore this research aims to discover new potential compounds that can inhibit HIV-1 and treat AIDS [2].
Zingiberaceae is a perennial herb that mostly grows in the subtropical and tropical climates of Asia and the Pacific [3]. Furthermore, this herb can be found in Sri Lanka, the Western Ghats of India, China, Japan, all of southeast Asia, and the Pacific, as far as Fiji, Samoa, the Caroline Islands, and Australia [4, 5]. There are about 1,200 species of Zingiberaceae in the world in which about 1000 occur in the tropical forest in Asia [4]. Indonesia, particularly the Sulawesi Island, has a biodiversity of Zingiberaceae plants such as Alpinia and Etlingera, which have been systematically studied by some botanists. Poulsen (2012) identified 48 taxa of Etlingera, and 36 of them were new species [6]. In addition, previous ethnopharmacology studies have identified three endemic Sulawesi's Zingiberaceae plants, namely Alpinia eremochlamys K. Schum, Etlingera flexuosa A.D. Poulsen, and Etlingera acanthoides A.D. Poulsen. These plants can be found in Lore Lindu National Park (LLNP) Central Sulawesi Indonesia and have been used traditionally by the Topo Baria ethnic group for medicine, tonicum, food flavoring, and food wrapping [7].
The use of Zingiberaceae plants as anti-HIV-1 agents has been studied, and it was discovered that methanol extract of Alpinia galanga rhizome showed potent inhibitory activity on HIV-1 PR. Furthermore, 19S-19-Acetoxychavicol acetate, which is isolated from Alpinia galanga, was reported to block Rev transport, therefore, inhibiting the replication of HIV type 1 [8,9,10]. (E)-Labda-8(17),12-diene-15,16-dial from Alpinia zerumbet has an inhibitive effect on HIV-integrase, while Dihydro-5,6-dehydrokawain and 5,6-dehydrokawain inhibit HIV-1 integrase with the respective IC50 values of 4.4 and 3.6 μg/mL [11, 12]. Zerumbone, the main compound from Zingiber zerumbet and Zingiber aromaticum, was also reported to inhibit HIV with an IC50 value of 0.04 μg/mL [13]. A new diarylheptanoid, (3S,5S)-3,5-diacetoxy-1,7-bis(3,4,5-trimethoxyphenyl) heptanes, along with 5αH-eudesmane-4α,11-diol, 5αH-eudesmane-4β,11-diol, 4α,10β-dihydroxy-1βH,5αH-guaia-6-ene (guaianediol), and (+)-galanolactone from the rhizomes of Zingiber mekongense also showed anti-HIV activities [14]. Therefore, in this study, the antiviral screening was performed on endemic Sulawesi's Zingiberaceae plants: Alpinia eremochlamys, Etlingera flexuosa, and Etlingera acanthoides (Figure 1), and the GC-MS profiles of the potential extracts were also discussed. This research is the first report regarding the antiviral activity of these plant extracts.
Figure 1.
A. eremochlamys with its flower and fruit (A), E. flexuosa with its flower (B), E. acanthoides with its rhizome (C).
2. Materials and methods
2.1. Chemicals
Ethanol (Merck, Darmstadt, Germany), Whatman 0.2 μm nitrocellulose membrane filter (Sigma-Aldrich, Singapore), Gibco RPMI-1640 medium (Thermo Fisher Scientific, USA), Gibco Fetal Bovine Serum (Thermo Fisher Scientific, USA), Sodium bicarbonate (Merck, Darmstadt, Germany), Viral-ToxGlo (catalog number G8943) was from Promega (Madison, WI, USA), Dimethyl sulfoxide (Sigma-Aldrich, Singapore), Sterile water for injection and aquadest.
2.2. Extraction
Alpinia eremochlamys, Etlingera flexuosa, and Etlingera acanthoides were collected from Lore Lindu National Park Central Sulawesi, Indonesia, in April 2019. The plants were identified by Ramadanil Pitopang (plant taxonomist) at the Laboratory of Plant Biosystematics/Herbarium Celebense (CEB), Tadulako University, and deposited with the specimen voucher number of 10042, 10041, and 10043, respectively. Each part of the plant (leaves, pseudostems, and rhizomes) was washed in running tap water, then cut into 3 cm pieces and dried at room temperature with no direct sunlight. After drying, about 2.0 kg of each part of the plant was extracted by maceration using 5 L of 96% ethanol for 3 × 24 h. The maceration was repeated three times, and then the filtrates were filtered and evaporated in a rotary evaporator to obtain the viscous extract. The extracts were then dissolved on 100% DMSO and diluted until obtain the series of sample concentrations with the final concentration of DMSO in each sample is 1%.
2.3. Cells and viruses
The MT-4 cells (ECACC 08081402) were obtained from the Institute of Tropical Disease (ITD) laboratory, Airlangga University, Surabaya, Indonesia. MT-4 cells were cultured in RPMI-1640 media and equipped with 10% FBS. The MT-4 cells were maintained in T25 CCF at 37 °C temperature in a 5% CO2 incubator. HIV was cultured on the MT-4 cell in RPMI-1640 medium completed with FBS 10%. MT-4/HIV cell was kept in CCF T25 at 37 °C in CO2 5% incubator. HIV isolates from a seropositive HIV donor labeled IDU-18 were obtained from ITD Laboratory, Airlangga University, Surabaya, Indonesia [15].
2.4. In vitro cytolysis activity
The in vitro cytolysis effect inhibitory test was performed by a colorimetric method using Viral-ToxGlo as our previous research with slight modification [15, 16]. MT-4 cells (2 × 105 cells/well), after infected with HIV, was placed in a 96-well microplate and then added by various concentrations of Alpinia eremochlamys, Etlingera flexuosa, and Etlingera acanthoides leaves, pseudostems, and rhizomes extracts (7.8, 15.6, 31.25, 62.5, 125, 250, 500, and 1000 μg/mL). The wells were incubated for 6 days at 37 °C temperatures in a 5% CO2 incubator. Then 10 μL Viral-ToxGlo was added to each well and was incubated for 60 min at a 37 °C temperature in a 5% CO2 incubator. The luminescence was measured using a multimode plate reader (GloMax Explorer Promega). The same procedure was applied on duviral (zidovudin and lamivudin) as positive control and 1% DMSO as a negative control. All experiments were performed in duplicates.
2.5. In vitro cytotoxicity test
The in vitro cytotoxicity test was done by a colorimetric method using Viral-ToxGlo as our previous research with slight modification [15, 16]. About 50 μL MT-4 cells in a 96-well microplate (2 × 105 cells/well) were added by various concentrations of Alpinia eremochlamys, Etlingera flexuosa and Etlingera acanthoides leaves, pseudostems and rhizomes extracts (7.8, 15.6, 31.25, 62.5, 125, 250, 500, and 1000 μg/mL). The wells were incubated for 6 days at 37 °C temperatures in a 5% CO2 incubator. Then 10 μL Viral-ToxGlo was added to each well and was incubated for 120 min at 37 °C temperature in a 5% CO2 incubator. The luminescence was measured using a multimode plate reader (GloMax Explorer Promega). The same procedure was applied on duviral (zidovudin and lamivudin) as positive control and 1% DMSO as a negative control. All experiments were performed in duplicates.
2.6. GC-MS analysis
The GC-MS analysis of sample extracts was carried out on a Shimadzu QP-2010 Ga Chromatograph Mass Spectrometer (GC-MS) Ultra, which is equipped with Autosampler AOC-20i and SH-Rxi-5Sil MS capillary column (30 m × 0.25 mm x 0.25 μm) using Helium as carrier gas (1.0 mL/min). The column temperature program was set as follows: an injection temperature of 250 °C, splitless mode, a column oven temperature of 70 °C at the beginning and held for 2 min, then ramped to 200 °C at the rate of 10 °C/min and end temperature 280 °C and held for 9 min at the rate 5 °C/min, an MS ion source temperature of 200 °C, and an interface temperature of 280 °C. The spectra for each of the chromatogram peaks were compared with the database library in the NIST and Wiley.
2.7. Statistical analysis
Data for 50% virus inhibitory concentration (IC50) and 50% cytotoxicity concentration (CC50) were analyzed by nonlinear regression analysis using GraphPad Prism® version 8.0.1 software. IC50 is the concentration that inhibits 50% of the viral cytopathic effect. CC50 is the concentration that generates 50% cytotoxicity on the MT-4 cells.
3. Results
3.1. Anti-HIV-1 activity
The anti-HIV-1 screening results showed that two of the nine tested extracts had promising inhibitory activity (Table 1). E. acanthoides rhizome extract was found to be very active with the IC50 value of 1.74 ± 2.46 μg/mL. Furthermore, it had less cytotoxicity on MT-4 cells with the CC50 value of 204.90 ± 106.35 μg/mL. Therefore, it had the highest selectivity index (SI) of 117.76. The rhizome extracts of A. eremochlamys also showed antiviral activity with the IC50 value of 64.18 ± 2.58 μg/mL, respectively. Meanwhile, this plant showed no toxicity on MT-4 cells. Therefore, it has high SI values of 19.05. Overall, The SI values of all extracts showed significant inhibition on the replication of HIV-1 in MT-4 cells (F = 122.66, p < 0.001). However, compared to positive control duviral, the inhibitory activity was significantly different.
Table 1.
IC50, CC50, and selectivity index (SI) of Alpinia eremochlamys, E. flexuosa and E. acanthoides ethanol extracts.
| Sample | IC50 (μg/mL) | CC50 (μg/mL) | SI |
|---|---|---|---|
| Alpinia eremochlamis | |||
| Leaf | 21.41 ± 24.26 | 9.87 ± 2.66 | 0.46a |
| Pseudostem | 67.96 ± 10.82 | 25.42 ± 6.53 | 0.37a |
| Rhizome | 64.18 ± 2.58 | 1223.05 ± 1469.29 | 19.05a |
| E. flexuosa | |||
| Leaf | 15.74 ± 2.95 | 2.45 ± 3.19 | 0.16a |
| Pseudostem | 106.75 ± 5.30 | 13.53 ± 0.28 | 0.13a |
| Rhizome | 20.31 ± 28.71 | 10.28 ± 3.28 | 0.51a |
| E. acanthoides | |||
| Leaf | 17.89 ± 12.33 | 18.64 ± 2.99 | 1.04a |
| Pseudostem | 29.08 ± 2.54 | 351.90 ± 131.80 | 12.10a |
| Rhizome | 1.74 ± 2.46 | 204.90 ± 106.35 | 117.76a |
| PC | 157.11 ± 174.63 | 1.28 × 1020 ± 1.81 × 1020 | 8.14 × 1017b |
| NC | 0 | 0 | 0 |
PC: Positive control (Duviral contained zidovudin and lamivudin); NC: Negative control (1% DMSO); n = 2.
Values = Mean ± SEM. Values with similar superscript letter are not significantly different with each other.
(p < 0.05), analyzed by ANOVA-Tukey's post-hoc multiple comparisons)
3.1.1. GC-MS analysis
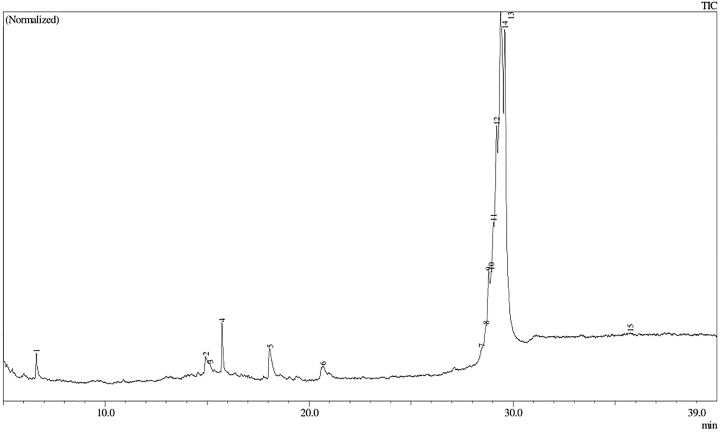
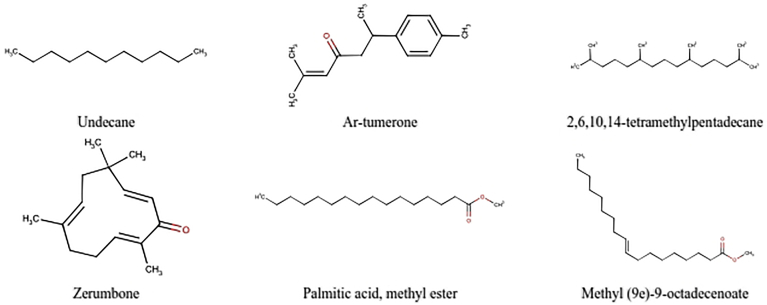
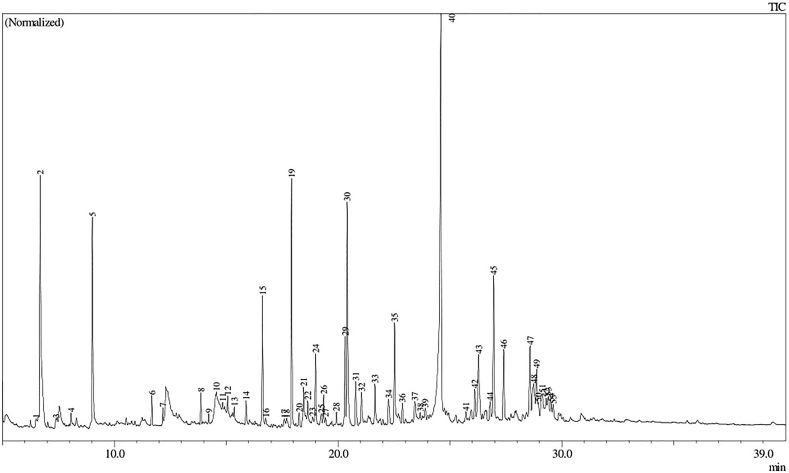
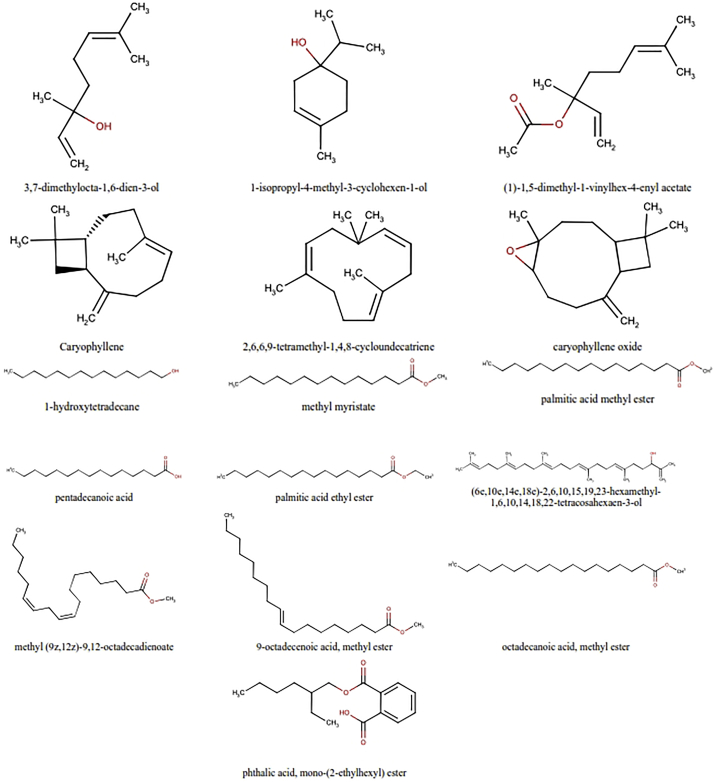
The list of metabolites on E. acanthoides and A. eremochlamys rhizomes identified by GC-MS can be seen in Table 2. The compounds was selected based on the high similarity index of their mass spectral with NIST and Wiley spectral databases (≥85%) and therefore, only 6 compounds were selected in E. acanthoides rhizome. It contained terpenoids of ar-tumerone (1.01%) and zerumbone (1.70%), long chain fatty acids methyl esters of palmitic acid (2.24%) and methyl (9E)-9-octadecenoate (0.94%), and hydrocarbon of undecane (0.94%) and 2,6,10,14-tetramethylpentadecane (0.38%) (Table 2 & Figures 2 and 3). Meanwhile, in the rhizomes of A. eremochlamys was selected 16 compounds in which fatty acids and terpenoids form the majority with a percentage of 23.26% and 17.67%, respectively. The terpenoids detected were 3,7-dimethylocta-1,6-dien-3-ol (9.46%), 1-isopropyl-4-methyl-3-cyclohexen-1-ol (0.29%), (1)-1,5-dimethyl-1-vinylhex-4-enyl acetate (5.51%), caryophyllene (0.61%), 2,6,6,9-tetramethyl-1,4,8-cycloundecatriene (0.31%), caryophyllene oxide (0.64%) and (6E,10E,14E,18E)-2,6,10,15,19,23-hexamethyl-1,6,10,14,18,22-tetracosahexaen-3-ol (0.85%). Meanwhile, the fatty acid consisted of saturated fatty acids such as methyl myristate (0.20%) and palmitic acid methyl ester (5.96%) and unsaturated fatty acids of methyl (9Z,12Z)-9,12-octadecadienoate (2.34%) and methyl ester 9-octadecenoic acid (6.94%) (Table 2 & Figures 4 and 5).
Table 2.
Gas Chromatography-Mass Spectroscopy (GC-MS) data of phytocompounds putatively identified in ethanol extract of A. eremochlamys and E. acanthoides rhizomes.
| No |
A. eremochlamis rhizome |
E.acanthoides rhizome |
||||
|---|---|---|---|---|---|---|
| Compounds | Peak area (%) | Similarity Index (%) | Compounds | Peak area (%) | Similarity Index (%) | |
| 1 | 1-cyclopentylacetone | 0.60 | 77 | Undecane | 0.94 | 95 |
| 2 | 3,7-dimethylocta-1,6-dien-3-ol | 9.46 | 95 | Ar-Tumerone | 1.01 | 90 |
| 3 | 1-isopropyl-1-methyl-2-oxohydrazine | 0.37 | 78 | 2,6,10,14-Tetramethylpentadecane | 0.38 | 85 |
| 4 | 1-isopropyl-4-methyl-3-cyclohexen-1-ol | 0.29 | 95 | Zerumbone | 1.70 | 92 |
| 5 | (1)-1,5-dimethyl-1-vinylhex-4-enyl acetate | 5.51 | 96 | Palmitic Acid, Methyl Ester | 2.24 | 94 |
| 6 | Caryophyllene | 0.61 | 97 | Methyl (9e)-9-Octadecenoate | 0.94 | 92 |
| 7 | 2,6,6,9-tetramethyl-1,4,8-cycloundecatriene | 0,31 | 96 | 1,2-Hexadecanediol | 0.84 | 59 |
| 8 | caryophyllene oxide | 0.64 | 91 | 2-Pentyl 6-(4-Pentylphenyl) 2,6-Naphthalenedicarboxylate | 1.54 | 55 |
| 9 | 2,5,9-trimethyl-4,8-cycloundecadien-1-one | 0.19 | 84 | 2-Pentyl 6-(4-Pentylphenyl) 2,6-Naphthalenedicarboxylate | 4.93 | 68 |
| 10 | ethyl iso-allocholate | 0.70 | 75 | 2-Pentyl 6-(4-Pentylphenyl) 2,6-Naphthalenedicarboxylate | 3.32 | 59 |
| 11 | 1-hydroxytetradecane | 0.17 | 94 | 2-Pentyl 6-(4-Pentylphenyl) 2,6-Naphthalenedicarboxylate | 6.81 | 65 |
| 12 | isochiapin b | 0.42 | 79 | 2-Pentyl 6-(4-Pentylphenyl) 2,6-Naphthalenedicarboxylate | 14.72 | 64 |
| 13 | methyl myristate | 0,20 | 93 | 2-Pentyl 6-(4-Pentylphenyl) 2,6-Naphthalenedicarboxylate | 38.17 | 66 |
| 14 | (7a-isopropenyl-4,5-dimethyl-octahydro-inden-4-yl)-methanol | 0,50 | 79 | 2-Pentyl 6-(4-Pentylphenyl) 2,6-Naphthalenedicarboxylate | 22.25 | 68 |
| 15 | 4-methyl-6-(2,6,6-trimethyl-1-cyclohexen-1-yl)-4-hexen-1-ol | 0,35 | 82 | 4-Tert-Butylphenoxy-.alpha.-Propionic Acid | 0.22 | 60 |
| 16 | 5-isopropenyl-2,7-dimethyl-1,8-nonadiene | 0.16 | 76 | |||
| 17 | 14b-pregnane | 0.26 | 79 | |||
| 18 | (7a-isopropenyl-4,5-dimethyloctahydroinden-4-yl)methanol | 0.27 | 82 | |||
| 19 | palmitic acid methyl ester | 5.96 | 95 | |||
| 20 | Methenolone | 0.39 | 78 | |||
| 21 | pentadecanoic acid | 1.53 | 88 | |||
| 22 | (4e)-4-methyl-6-(2,6,6-trimethyl-1-cyclohexen-1-yl)-4-hexen-1-ol | 1.24 | 82 | |||
| 23 | palmitic acid ethyl ester | 0.54 | 90 | |||
| 24 | 1,5,9-trimethyl-12-(1-methylethyl)- 4,8,13-cyclotetradecatriene-1,3-diol | 2.12 | 77 | |||
| 25 | isopropyl palmitate | 0.53 | 76 | |||
| 26 | (6e,10e,14e,18e)-2,6,10,15,19,23-hexamethyl-1,6,10,14,18,22-tetracosahexaen-3-ol | 0.85 | 91 | |||
| 27 | (4e)-4-methyl-6-(2,6,6-trimethyl-1-cyclohexen-1-yl)-4-hexen-1-ol | 0.20 | 81 | |||
| 28 | alloaromadendrenoxid-(1) | 0.32 | 79 | |||
| 29 | methyl (9z,12z)-9,12-octadecadienoate | 2.34 | 94 | |||
| 30 | 9-octadecenoic acid, methyl ester | 6.94 | 90 | |||
| 31 | octadecanoic acid, methyl ester | 1.29 | 94 | |||
| 32 | isoretinene a | 1.11 | 71 | |||
| 33 | (7a-isopropenyl-4,5-dimethyl-octahydro-inden-4-yl)-methanol | 1.07 | 82 | |||
| 34 | andrographolide | 0.99 | 76 | |||
| 35 | selina-3,7(11)-dien | 2.92 | 71 | |||
| 36 | dodecahydro-3,8,8,11a-tetramethyl-5h-3,5a-epoxynaphth[2,1-c]oxepin | 0.56 | 76 | |||
| 37 | longifolenaldehyd | 0.90 | 79 | |||
| 38 | (albicanol) decahydro-2-methylene-5,5,8a-trimethyl-1-naphthalenemethanol | 0.37 | 77 | |||
| 39 | (albicanol) decahydro-2-methylene-5,5,8a-trimethyl-1-naphthalenemethanol | 0.30 | 75 | |||
| 40 | alloaromadendrenoxid-(1) | 19.7 | 79 | |||
| 41 | Strophanthidol | 0.26 | 76 | |||
| 42 | (albicanol) decahydro-2-methylene-5,5,8a-trimethyl-1-naphthalenemethanol | 0.93 | 74 | |||
| 43 | 19,19-dimethoxy-3-oxoandrost-1-en-17-yl acetate | 3.09 | 78 | |||
| 44 | 2,4a,8,8-tetramethyl-decahydro-4cyclopropa[d]naphthalene | 0.59 | 75 | |||
| 45 | phthalic acid, mono-(2-ethylhexyl) ester | 4.46 | 95 | |||
| 46 | (4e)-4-methyl-6-(2,6,6-trimethyl-1-cyclohexen-1-yl)-4-hexen-1-ol | 2.36 | 73 | |||
| 47 | (4ar,9as,9bs)-4a,6,6,9a-tetramethyl-trans-perhydroindano[2,1-c]pyran | 2.75 | 74 | |||
| 48 | 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-butanone | 2.37 | 67 | |||
| 49 | 4,4-dimethylandrost-5-en-3-one | 1.65 | 67 | |||
| 50 | 5α,17α,20α-cholestane-3β,6α-diol | 0.42 | 58 | |||
| 51 | 2,6,10,15,19,23-hexamethyl-2,6,10,14,18,22-tetracosahexaene | 1.73 | 74 | |||
| 52 | 2,6,10,15-tetramethyl-17-(1,4,4-trimethyl-cyclohex-2-enyl)-heptadeca-2,6,10,14-tetraene | 1.01 | 71 | |||
| 53 | 1-heptatriacontanol | 1.08 | 67 | |||
| 54 | pregn-20-yn-17-ol | 0.61 | 74 | |||
| 55 | pregn-20-yn-17-ol | 0.50 | 75 | |||
| Total Indentification (%) | 95.63 | 100.01 | ||||
Figure 2.
GC-MS chromatogram of the ethanol extract of E. acanthoides rhizome.
Figure 3.
Secondary metabolites identified on E. acanthoides rhizome ethanol extract by GC-MS.
Figure 4.
GC-MS chromatogram of the ethanol extract of A. eremochlamis rhizome.
Figure 5.
Secondary metabolites identified on A. eremochlamys rhizome ethanol extract by GC-MS.
4. Discussions
Zingiberaceae plants, in individual or mixed combination, was popular to be used by local people in Indonesia on treating several disease such as fever, cough, and stomach ache. The ethnopharmacology study of Zingiberaceae plants collected in Central Sulawesi, found the application of Curcuma longa to treat the HIV-1 infection. Literature investigation found that Curcuma longa contained curcumin as major compound that has been reported to possess a wide range inhibition of viruses such as dengue virus, herpes virus, and human immunodeficiency virus [7, 17]. Therefore, we interest in further testing the other Zingiberaceae plants for antiviral activity.
In this study, the antiviral screening was performed to examine the inhibitory effects of three endemic Zingiberaceae plants: Alpinia eremochlamys, Etlingera flexuosa, and Etlingera acanthoides (their leaves, pseudostems, and rhizomes) on the replication of HIV-1 in MT-4 cells. These plants were collected from the montane forest of Lore Lindu National Park (LLNP), Central Sulawesi, Indonesia. It is an area with about 220,000 ha of land and a habitat for Wallacean plants and animals, considered as a protected conservation area. These endemic plants can be differentiated from each other by comparing their morphology, such as the flower and the rhizome [3, 6]. All plants were extracted using 96% ethanol which is categorized as a polar and non-toxic solvent.
The inhibitory effect of these extracts on HIV-1 replication was estimated and monitored by a Viral-ToxGlo assay and their virus-induced cytopathic effect on MT-4 cells. Furthermore, the cytotoxicity of the extracts on MT-4 cells was also assessed using the Viral-ToxGlo assay, which is known for high throughput antiviral screening [18]. This assay measures cellular ATP, which is used to determine the viability of the host cell. The screening results showed that rhizome parts of the plants showed more potent inhibitory activity than leaves and pseudostems parts. The ethanol extract of E. acanthoides rhizome was the most potent extract with the IC50 value of 1.74 ± 2.46 μg/mL and the highest selectivity index (SI) of 117.76. A. eremochlamys rhizome ethanol extract was also exhibited potent activity with the IC50 of 64.18 ± 2.58 μg/mL and high SI of 19.05. Meanwhile, the ethanol extract of E. flexuosa rhizome did not show remarkable inhibitory activity assigned by the low SI of 0.51. The selectivity index was obtained by dividing the CC50 value by its IC50 value. Therefore, a higher SI ratio in an extract will mean a stronger inhibitory effect on viral replication in vitro [19]. This result highlighted the potency of E. acanthoides and A. eremochlamys rhizomes as sources of anti-HIV-1 drugs.
Till date, there is still no report regarding the antiviral activity of Etlingera plants. However, Etlingera elatior, the most studied species in this genus, has been reported as an antibacterial, antifungal, anticancer, hepato-protector, and tyrosinase inhibitor [20]. Besides that, the docking simulation of a metabolite from E. elatior identified a steroid compound, 5α,8α-Epidioxyergosta-6,22-dien-3β-ol, which has an inhibitory effect on HIV-1 integrase enzyme [21]. Meanwhile, Alpinia plants such as Alpinia galanga and Alpinia zerumbet were reported as anti-HIV drug sources as most of their bioactive components consisted of terpenoids and phenolic type compounds [11].
Preliminary identification via GC-MS was done to identify the chemical contents of the extracts of E. acanthoides and A. eremochlamys rhizomes using the NIST and Wiley databases library as a reference. E. acanthoides rhizomes contained a terpenoid compound, zerumbone, which is reported to possess anti-HIV-1 activity [13]. Furthermore, it contains ar-turmerone which is a major component of Curcuma longa and has biological activity as an anti-tumor, anti-inflammatory and neurological agent [22, 23, 24, 25, 26].
3,7-dimethylocta-1,6-dien-3-ol was found as the major terpenoid in the rhizomes of A. eremochlamys with a high percentage of 9.46%. In addition, the sesquiterpenoids caryophyllene and caryophyllene oxide were also detected with percentage of 0.61% and 0.64%, respectively. Caryophyllene and caryophyllene oxide were essential oils that mostly found in numerous higher plants. They are reported to possess anti-inflammatory, antifungal, and anti-tumor. Caryophyllene also reported to exhibit antiviral activity on herpes simplex virus with high selectivity index [27, 28, 29].
Saturated and unsaturated long-chain fatty acids were also detected on these two rhizome extracts. Among them, methyl ester of palmitic acid was found abundant on E.acanthoides and A.eremochlamys rhizome extract with the concentration of 2.24% and 5.96%, respectively. Fatty acids were recognized to possess antiviral activity. Palmitic acid (saturated) and oleic acid (unsaturated) combination treatment on hepatitis C virus (HCV) cell culture can induce ER stress and blocking the antiviral activity of interferon alpha [30]. Further study on the isolation of pure compounds of these potential plants and testing their antiviral activity are necessary. However, this report provides scientific justification for the application of these plants as antiviral medicinal herbs.
5. Conclusion
The screening of antiviral activity showed that the ethanol extract of E. acanthoides and A. eremochlamys rhizomes have the potency to inhibit the replication of HIV-1 on MT-4 cells in vitro. E. acanthoides rhizome showed the best antiviral activity with the lowest IC50 value of 1.74 ± 2.46 μg/mL, less cytotoxicity on MT-4 cells, and the highest selectivity index of 117.76. The preliminary identification of chemical constituents by GC-MS found terpenoids such as zerumbone, ar-turmerone, caryophyllene, and caryophyllene oxide and also several saturated and unsaturated fatty acids that might be responsible for the antiviral activity of these plants.
Declarations
Author contribution statement
Muhammad Sulaiman Zubair: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Siti Qamariyah Khairunisa: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Agustinus Widodo: Performed the experiments; Analyzed and interpreted the data.
Nasronudin: Analyzed and interpreted the data.
Ramadanil Pitopang: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Ministry of Research and Technology, the Republic of Indonesia (301/UN28.2/PL/2020).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The Agency of Lore Lindu National Park (BBTNLL) for permission to collect sample materials.
References
- 1.UNAIDS . Feb. 20, 2021. Global HIV & AIDS Statistics – 2020 Fact Sheet.https://www.unaids.org/en/resources/fact-sheet [Online]. Available: [Google Scholar]
- 2.Arslan N. Molecular docking study of four chromene derivatives as novel HIV-1 integrase inhibitors. J. Turk. Chem. Soc. Sect. Chem. Mar. 2019:133–142. [Google Scholar]
- 3.Pitopang R., Umrah U., Harso W., Nurainas N., Zubair M.S. Some botanical aspects of Etlingera flexuosa (Zingiberaceae) from Central Sulawesi (Indonesia) and its antifungal activity. Biodiversitas J. Biol. Divers. Jul. 2020;21(8) [Google Scholar]
- 4.Larsen K., Ibrahim H., Khaw S., Saw L. Natural History Publications (Borneo); 1999. Gingers of Peninsular Malaysia and Singapore. [Google Scholar]
- 5.Oyen L., Dung N. Prosea Foundation, Backhuys Publisher; Leiden, The Netherlands: 1990. Plant Resources of South East Asia: Essential Oil Plants. [Google Scholar]
- 6.Poulsen A. Natural History Publications (Borneo) Sdn. Bhd.; Kota Kinabalu: 2012. Etlingera of Sulawesi. [Google Scholar]
- 7.Pitopang R., Damry D., Rusdi R., Hamzah B., Zubair M.S. Traditional usages and phytochemical screenings of selected Zingiberaceae from central Sulawesi, Indonesia. Pharm. J. May 2019;11(3):505–510. [Google Scholar]
- 8.Sookkongwaree K., Geitmann M., Roengsumran S., Petsom A., Danielson U.H. Inhibition of viral proteases by Zingiberaceae extracts and flavones isolated from Kaempferia parviflora. Pharm. Times. Aug. 2006;61(8):717–721. [PubMed] [Google Scholar]
- 9.Tewtrakul S., Subhadhirasakul S., Kummee S. HIV-1 protease inhibitory effects of medicinal plants used as self medication by AIDS patients. Songklanakarin J. Sci. Technol. May 2003:6. [Google Scholar]
- 10.Ye Y., Li B. “1’S-1’-acetoxychavicol acetate isolated from Alpinia galanga inhibits human immunodeficiency virus type 1 replication by blocking Rev transport. J. Gen. Virol. Jul. 2006;87(Pt 7):2047–2053. doi: 10.1099/vir.0.81685-0. [DOI] [PubMed] [Google Scholar]
- 11.Ma X.-N., Xie C.-L., Miao Z., Yang Q., Yang X.-W. An overview of chemical constituents from Alpinia species in the last six decades. RSC Adv. 2017;7(23):14114–14144. [Google Scholar]
- 12.Upadhyay A., Chompoo J., Kishimoto W., Makise T., Tawata S. HIV-1 integrase and neuraminidase inhibitors from Alpinia zerumbet. J. Agric. Food Chem. Apr. 2011;59(7):2857–2862. doi: 10.1021/jf104813k. [DOI] [PubMed] [Google Scholar]
- 13.Dai J.-R., Cardellina J.H., Mahon J.B.M., Boyd M.R. Zerumbone, an HIV-inhibitory and cytotoxic sesquiterpene of Zingiber aromaticum and Z. zerumbet. Nat. Prod. Lett. May 1997;10(2):115–118. [Google Scholar]
- 14.Chareonkla A. A new diarylheptanoid from the rhizomes of Zingiber mekongense. Fitoterapia. Jun. 2011;82(4):534–538. doi: 10.1016/j.fitote.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Widodo A., Widiyanti P., Prajogo B. “Antiviral Activity of Justicia gendarussa Burm.f. leaves against HIV-Infected MT-4 cells,” Afr. J. Infect. Dis. 2018;12(1 Suppl):36–43. doi: 10.2101/Ajid.12v1S.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smee D.F. Evaluation of cell viability dyes in antiviral assays with RNA viruses that exhibit different cytopathogenic properties. J. Virol. Methods. Aug. 2017;246:51–57. doi: 10.1016/j.jviromet.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jennings M.R., Parks R.J. Curcumin as an antiviral agent. Viruses. Oct. 2020;12(11) doi: 10.3390/v12111242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maddry J.A. Discovery of novel benzoquinazolinones and thiazoloimidazoles, inhibitors of influenza H5N1 and H1N1 viruses, from a cell-based high-throughput screen. J. Biomol. Screen. Jan. 2011;16(1):73–81. doi: 10.1177/1087057110384613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pritchett J., Naesens L., Montoya J. Chapter 19 - treating HHV-6 infections: the laboratory efficacy and clinical use of anti-HHV-6 agents. In: Flamand L., editor. Elsevier BV; Amsterdam: 2014. (Human Herpesviruses HHV-6A, HHV-6B & HHV-7 (third ed.), Diagnosis and Clinical Management). [Google Scholar]
- 20.Chan E.W.C., Lim Y.Y., Wong S.K. Phytochemistry and pharmacological properties of Etlingera elatior: a review. Pharm. J. Jun. 2011;3(22):6–10. [Google Scholar]
- 21.Zubair M.S., Maulana S., Widodo A., Mukaddas A., Pitopang R. Docking study on anti-HIV-1 activity of secondary metabolites from Zingiberaceae plants. J. Pharm. BioAllied Sci. 2020;12(6):763. doi: 10.4103/jpbs.JPBS_261_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee Y. Activation of apoptotic protein in U937 cells by a component of turmeric oil. BMB Rep. Feb. 2009;42(2):96–100. doi: 10.5483/bmbrep.2009.42.2.096. [DOI] [PubMed] [Google Scholar]
- 23.Park S.Y., Kim Y.H., Kim Y., Lee S.-J. Aromatic-turmerone attenuates invasion and expression of MMP-9 and COX-2 through inhibition of NF-κB activation in TPA-induced breast cancer cells. J. Cell. Biochem. Dec. 2012;113(12):3653–3662. doi: 10.1002/jcb.24238. [DOI] [PubMed] [Google Scholar]
- 24.Park S.Y., Jin M.L., Kim Y.H., Kim Y., Lee S.J. Anti-inflammatory effects of aromatic-turmerone through blocking of NF-κB, JNK, and p38 MAPK signaling pathways in amyloid β-stimulated microglia. Int. Immunopharm. Sep. 2012;14(1):13–20. doi: 10.1016/j.intimp.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Park S.Y., Kim Y.H., Kim Y., Lee S.-J. “Aromatic-turmerone’s anti-inflammatory effects in microglial cells are mediated by protein kinase A and heme oxygenase-1 signaling. Neurochem. Int. Oct. 2012;61(5):767–777. doi: 10.1016/j.neuint.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 26.Hucklenbroich J. Aromatic-turmerone induces neural stem cell proliferation in vitro and in vivo. Stem Cell Res. Ther. 2014;5(4):100. doi: 10.1186/scrt500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fidyt K., Fiedorowicz A., Strządała L., Szumny A. β -caryophyllene and β -caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. Oct. 2016;5(10):3007–3017. doi: 10.1002/cam4.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amiel E., Ofir R., Dudai N., Soloway E., Rabinsky T., Rachmilevitch S. β -caryophyllene, a compound isolated from the biblical balm of gilead (Commiphora gileadensis), is a selective apoptosis inducer for tumor cell lines. Evid. Based Complement. Alternat. Med. 2012;2012:1–8. doi: 10.1155/2012/872394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Astani A., Reichling J., Schnitzler P. “Screening for antiviral activities of isolated compounds from essential oils,” evid. Based complement. Alternative Med. 2011;2011:1–8. doi: 10.1093/ecam/nep187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunduz F. Free fatty acids induce ER stress and block antiviral activity of interferon alpha against hepatitis C virus in cell culture. Virol. J. 2012;9(1):143. doi: 10.1186/1743-422X-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.