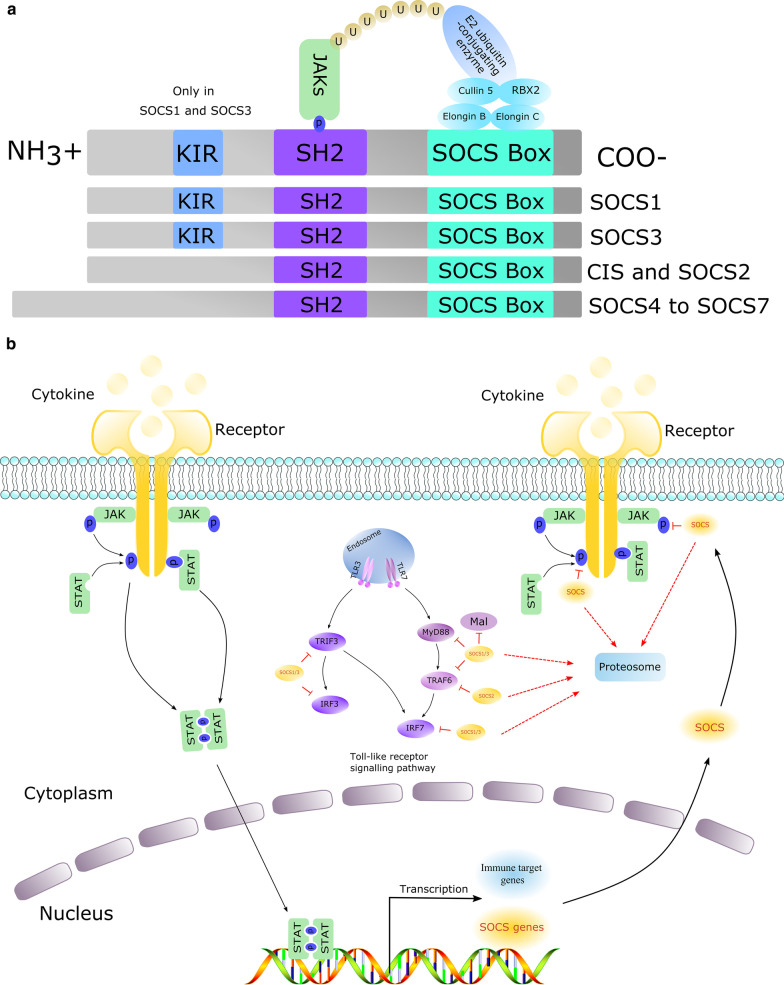
Fig. 1.
a SOCS protein structure. All SOCS proteins contain a central SH2 domain, an amino-terminal domain of variable length and a carboxy-terminal SOCS box. The SH2 domain recognizes and binds phosphorylated tyrosine residues on its specific substrate, such as JAK proteins. The SOCS box can interact with elongin B, elongin C, and cullin 5, utilizes the RING-finger-domain-only protein to recruit E2 ubiquitin-transferase, and ubiquitinates JAKs and other cytokine receptors, ultimately targeting them for proteasomal degradation. b Mechanism by which the SOCS proteins suppress the JAK-STAT pathway and TLR signaling pathway. Cytokines or interferons bind cellular membrane surface receptors, which activate and phosphorylate receptor-associated JAK proteins. Activated JAK proteins phosphorylate receptor cytoplasmic domains, which begin to recruit STATs, enabling their dimerization. Next, the dimerized complex enters the nucleus to initiate the transcription of different target genes, including the SOCS gene and immune effectors. SOCS proteins negatively regulate these pathways. In the JAK-STAT pathway, SOCS proteins compete with recruited STAT proteins for shared phospho-tyrosine residues or inhibit the activity of JAKs by the KIR domain of SOCS1 and SOCS3. Additionally, the SOCS box mediates the ubiquitination and degradation of bound receptor components. In the Toll-like receptor signaling pathway, SOCS1 and SOCS3 use the SH2 region to recognize and bind tyrosine-phosphorylated Mal, TNF receptor-associated factor 3/6 (TRAF3/6) and IRF7 [12, 17, 22]