Figure 1.
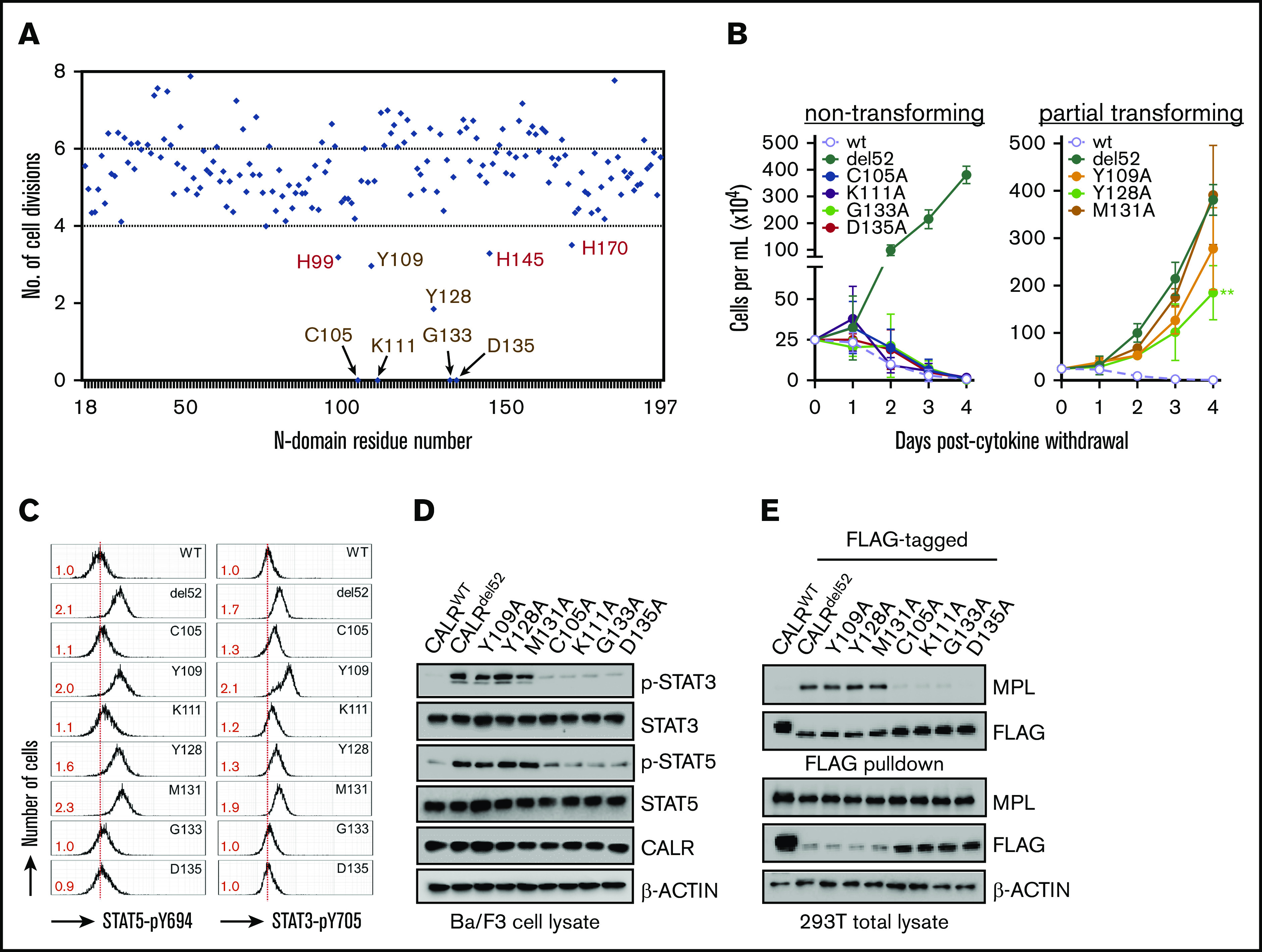
The lectin-dependent function of CALRdel52is required for cytokine-independent growth. (A) Alanine mutagenesis screen to assess ability of 177 CALRdel52 single-residue mutants to confer cytokine-independent growth of Ba/F3-MPL cells, as depicted on y-axis by number of cell divisions following 3 days. The mutagenized residue is represented on the x-axis. The 9 residues that are >1 standard deviation away from mean are indicated with arrows. Putative lectin residues are denoted in brown; putative zinc-binding residues are denoted in red. (B) Growth curves of Ba/F3-MPL cells expressing wild-type CALR, CALRdel52, or lectin motif variants demonstrate total (left) or partial (right) impairment of cytokine-independent growth. Testing for statistical significance was performed using a Student t test (*P < .05; **P < .01; ***P < .001). (C) Intracellular phosphorylation flow cytometry and (D) Western immunoblotting demonstrate diminished Stat3 and Stat5 phosphorylation in Ba/F3-MPL cells expressing nontransforming CALRdel52 lectin variants. Numbers in red indicate ratio of mean fluorescence intensity for each sample relative to isotype control. The data are representative of 2 independent experiments. (E) Immunoblotting of FLAG-immunoprecipitated proteins from 293T cells cotransfected with FLAG-tagged CALRdel52 lectin variants and MPL-expressing vector demonstrates impaired MPL binding capacity by nontransforming lectin variants.
