Figure 3.
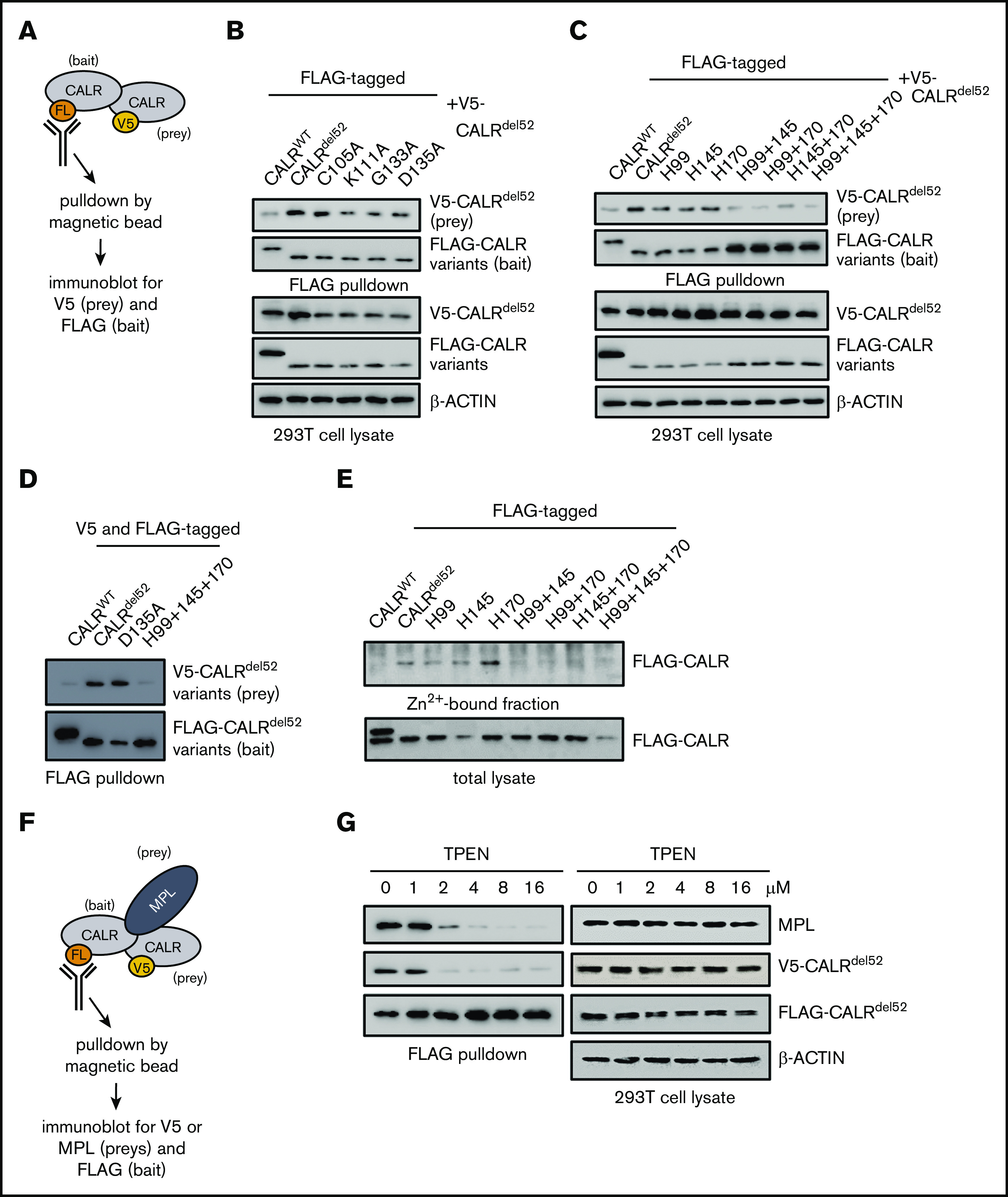
Zinc binding by CALRdel52is required for homomultimerization and enables MPL activation. (A) Schematic depicting co-immunoprecipitation assay for detecting FLAG-tagged and V5-tagged CALRdel52 multimers. (B) FLAG-pulldown assays demonstrating CALRdel52 lectin variants retain ability to bind to V5-CALRdel52. (C) FLAG-pulldown assays demonstrating 2His- and 3His-CALRdel52 are compromised in ability to bind V5-CALRdel52 compared with 1His-CALRdel52. (D) FLAG-pulldown assays of cells transfected with FLAG- and V5-tagged CALRWT (lane 1), CALRdel52 (lane 2), CALRdel52 D135A lectin variant (lane 3), or 3His-CALRdel52 variant (lane 4) demonstrating that CALRdel52 and CALRdel52 D135A are capable of homomultimerization, but not CALRWT and 3His-CALRdel52. (E) Affinity chromatography demonstrating 2His- and 3His-CALRdel52 bind less efficiently to zinc resin compared with 1His-CALRdel52. (F) Schematic depicting co-immunoprecipitation assay for detecting heteromeric complexes comprising FLAG-tagged and V5-tagged CALRdel52 proteins and MPL. (G) FLAG-pulldown assays demonstrating zinc chelator TPEN treatment disrupts CALRdel52 multimerization and MPL binding in 293T cells.
