Figure 5.
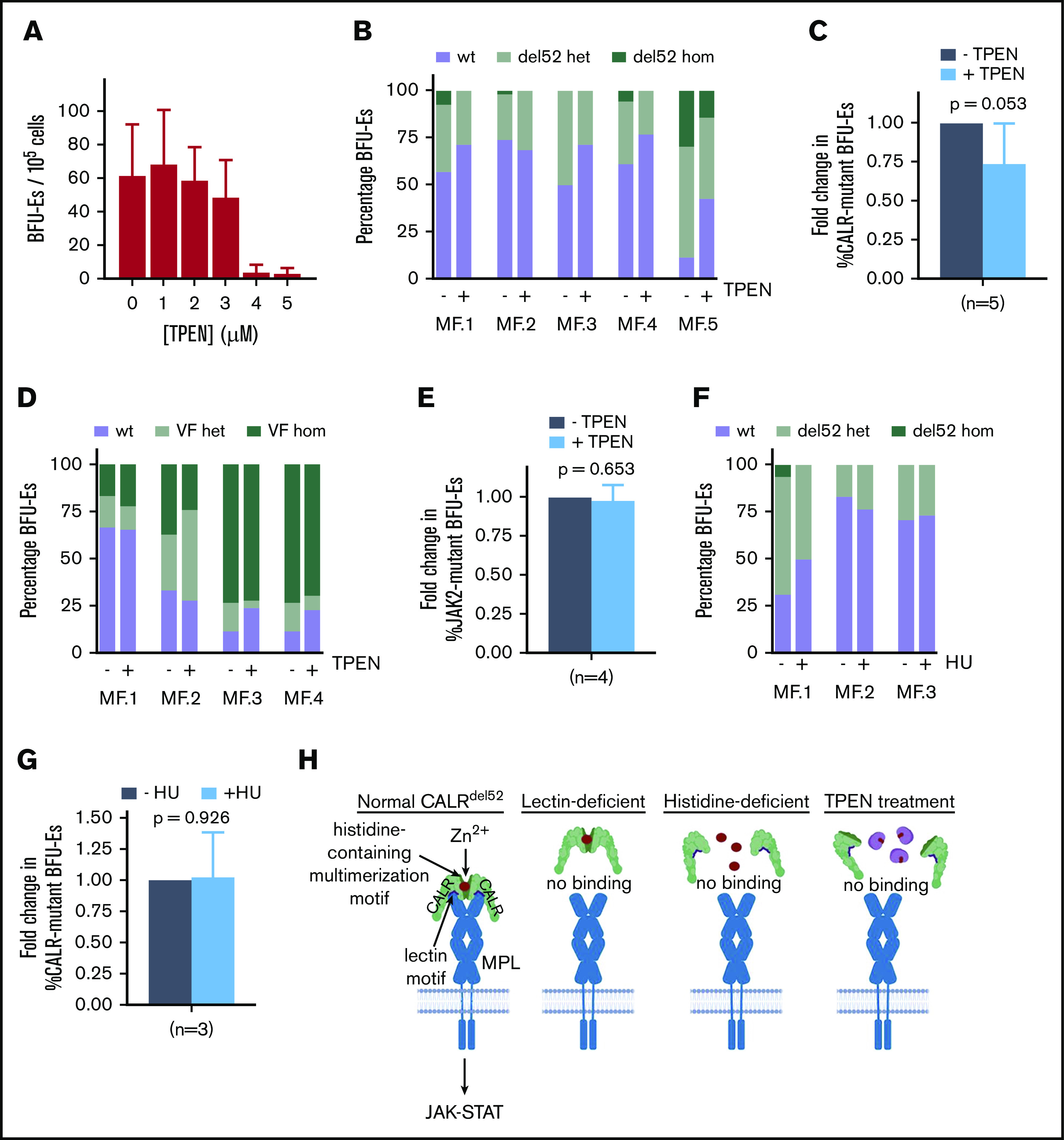
Zinc chelation exhibits selective cytotoxicity against CALR-mutant cells from MPN patients. (A) BFU-E colony counts of PBMCs from healthy volunteers grown in methylcellulose in the presence of 0 to 5 μM TPEN. (B) PBMCs from 5 myelofibrosis patients (allele burden ranging from 20% to 73%) were cultured in the presence and absence of 2.5 μM TPEN, and BFU-Es genotyped for the presence of CALR del52 mutation after 14 days. Proportion of colonies with wild-type CALR (wt), heterozygous for CALR del52 mutation (del52 het), or homozygous for CALR del52 mutation (del52 hom) cultured in the presence and absence of 2.5 μM TPEN. At least 20 colonies were genotyped for each patient. (C) Average fold change of CALR-mutant colonies (including heterozygous and homozygous colonies) for 5 MF patients examined following TPEN treatment relative to untreated cultures, which was arbitrarily designated as 1. (D) Proportion of colonies with JAK2 (wt), heterozygous for JAK2V617F mutation (VF het), or homozygous for JAK2V617F mutation (VF hom) cultured in the presence and absence of 2.5 μM TPEN. At least 20 colonies were genotyped for each patient. (E) Average fold change of JAK2-mutant colonies (including heterozygous and homozygous colonies) for 4 MF patients examined following TPEN treatment relative to untreated cultures, which was arbitrarily designated as 1. (F) Proportion of colonies with CALR (wt), heterozygous for CALR del52 mutation (del52 het), or homozygous for CALR del52 mutation (del52 hom) cultured in the presence and absence of 2 μM hydroxyurea (HU). At least 15 colonies were genotyped for each patient. (G) Average fold change of CALR-mutant colonies (including heterozygous and homozygous colonies) for 3 MF patients examined following HU treatment relative to untreated cultures, which was arbitrarily designated as 1. (H) Model depicting interplay between zinc, CALRdel52 multimerization and MPL activation. (C-G) Testing for statistical significance was performed using Student t test (*P < .05).
