Figure 9.
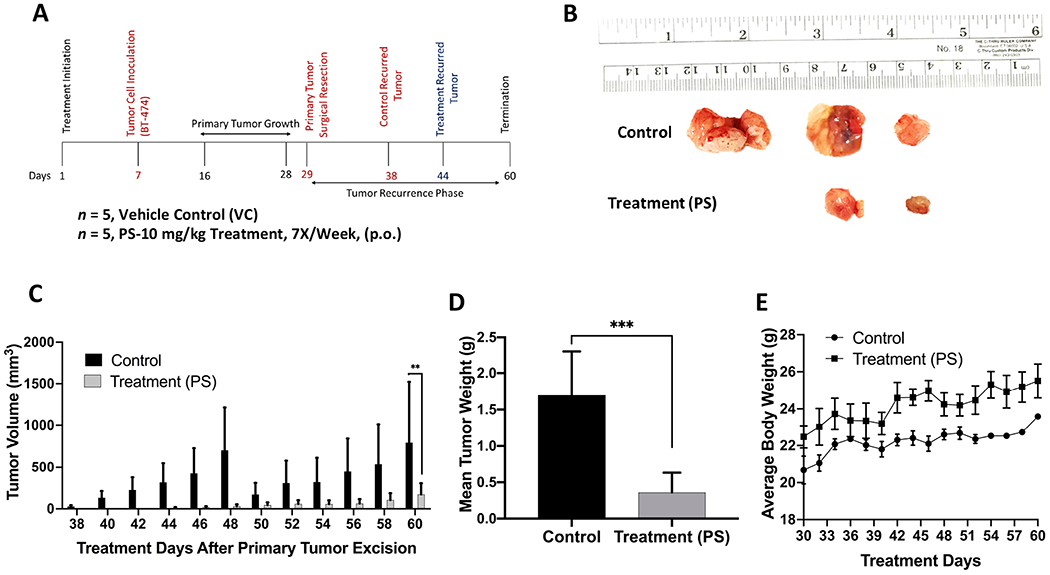
Recurrence suppressive effects of oral PS treatment after BT-474 BC cells orthotopically xenografted in athymic nude mice, allowed to grow tumors, followed by primary tumor surgical excision. (A) Overview of the experimental design. (B) Collected recurred tumors of each experimental group after the experiment completion. The top row shows the vehicle-treated control group recurred tumors (3 out of 5), while the bottom row shows tumors collected from PS-treated mice, 10 mg/kg, oral, 7X/week (2 out of 5). (C) Comparative monitoring of the mean recurrence tumor volumes for PS versus vehicle control-treated mice over the recurrence experiment course. Bars represent the mean tumor volume; error bars represent the SEM for each experimental group. **p<0.01 for statistical significance comparing to vehicle-treated control group. (D) Comparison of the mean recurred tumors weights for PS-treated versus vehicle control-treated mice at the experiment end. Error bars indicate SEM. ***p <0.001 for statistical significance. (E) Mice body weight-monitoring data over the experiment course. Points represent the mean body weights for animals in each group. Error bars indicate SEM.
