Figure 3. Representative results for the NADH dehydrogenase assay to test outer membrane (OM) purity.
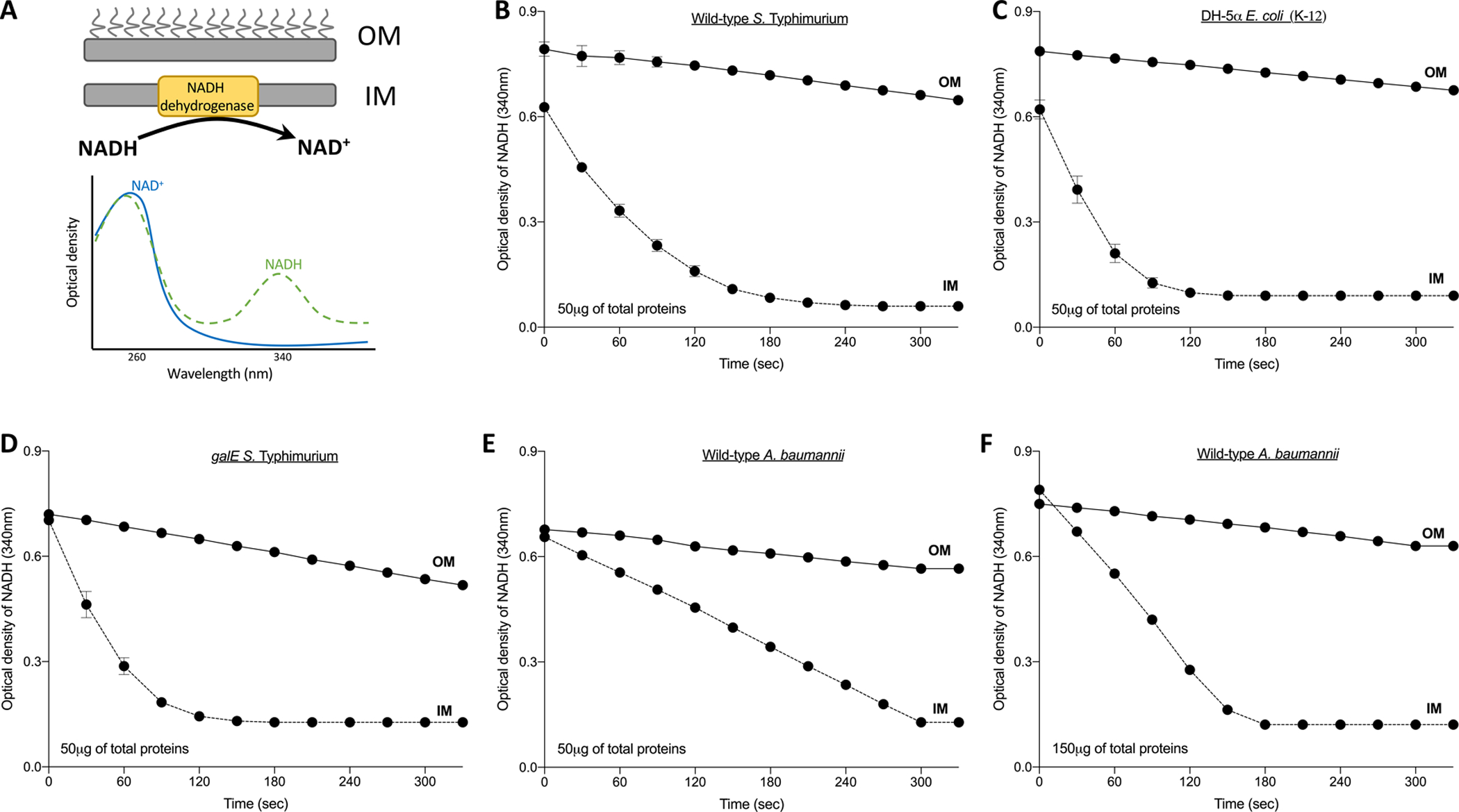
The presence of the enzyme, NADH-dehydrogenase, was tested in inner (IM) and OM samples to test the efficiency of the separation. A. The oxidation of NADH to NAD is catalyzed by an enzyme located in the bacterial IM. The reaction substrate (NADH) has a maximum absorbance at 340 nm; therefore, a decrease in optical density at this wavelength is indicative of the presence of the enzyme in the sample. The IMs and OMs were measured for B. wild-type S. Typhimurium, C. E. coli K-12 DH5α and D. galE-mutant S. Typhimurium. These membranes were isolated using an isopycnic sucrose density gradient of 20%/53%/73% w/v sucrose. For A. baumannii, the membranes were isolated using a gradient of 20%/45%/73% w/v sucrose. The NADH assay to test the purity of membranes was done using E. 50 μg for the enterobacterial organisms and F. 150μg of total proteins for A. baumannii. A higher concentration of protein was added in F., since the curve for E. suggested that the relative levels of NADH dehydrogenase compared to total protein were less for A. baumannii than for S. Typhimurium and E. coli.
