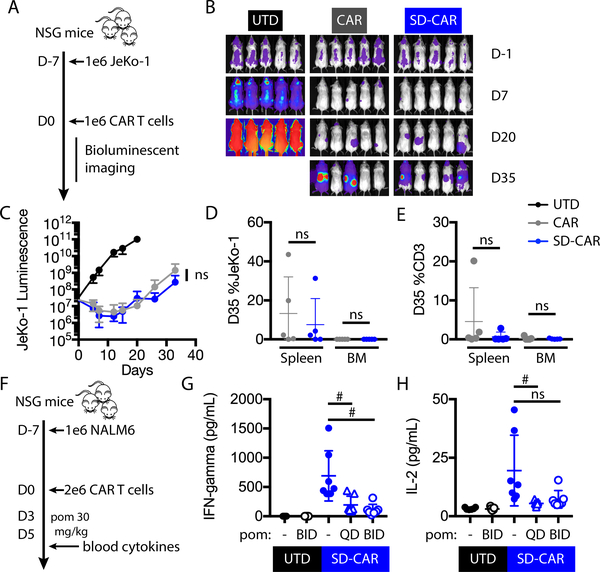
Fig. 7: Degradable CAR function and cytokine OFF-switch in vivo.
(A) Experimental design: NSG mice were injected intravenously with 1 × 106 GFP+/luciferase+ JeKo-1 tumor cells. At day 0, mice were randomly assigned on the basis of tumor burden to receive 1 × 106 untransduced, CAR, or SD-CAR T cells. (B) Bioluminescence of whole mice in each group, indicating tumor burden. (C) Bioluminescence flux (photons/s) at representative time points. Two-way ANOVA, ns, P > 0.05. (D, E) Percent of JeKo-1 and human CD3+ cells in the bone marrow and spleen at day 35 (D35). Error bars indicate mean ± SD of pentuplicate groups. (F) Experimental design for in vivo CAR T cell cytokine release analysis: NSG mice were injected intravenously with 1 × 106 NALM6 cells. At day 0, mice were randomly assigned on the basis of tumor burden to receive 2 × 106 untransduced or SD-CAR T cells. From days 3 – 5, mice received no treatment, once daily, or twice daily 30 mg/kg pomalidomide by oral gavage. On the afternoon of day 5, serum was analyzed by bead array for human cytokines. (G, H) Serum IFN-γ and IL-2 concentrations. QD, daily. BID, twice daily. Two-way t-test, # P < 0.05; ns, P > 0.05.