Figure 3. Metabolic alterations and vulnerabilities of RAS-driven cancers.
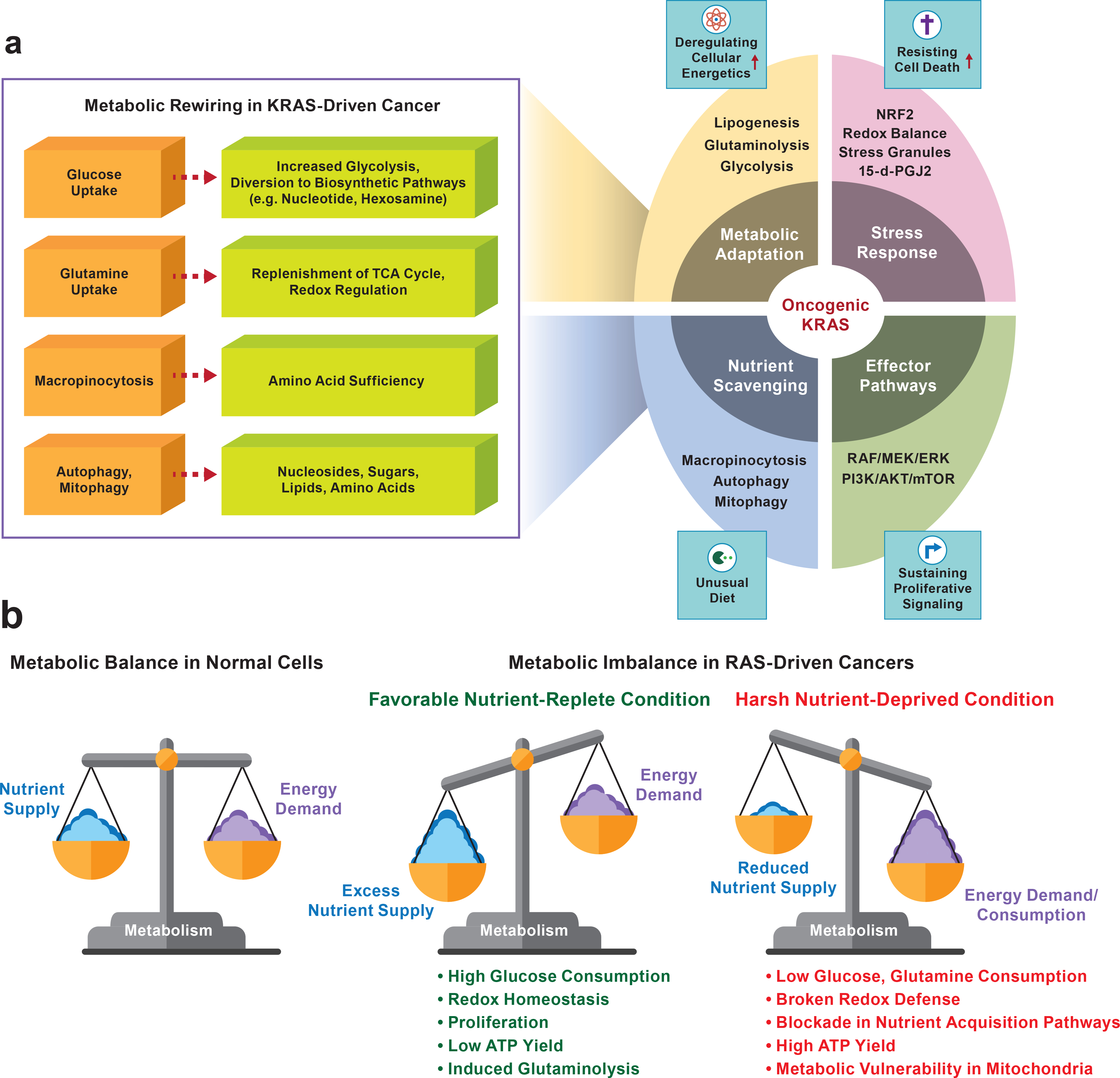
a. Schematic representation of the impact of oncogenic KRAS on cancer metabolism. Various metabolic pathways involving KRAS-mutants critical in cancer cell proliferation and cell survival. Increased glucose uptake, induced glutaminolysis, autophagy and micropinocytosis are involved in deregulating energetics and nutrient scavenging which results in RAS-driven cancer cells’ metabolic adaption for the benefit of cell growth. b. The delicate balance of nutrient supply and demand dynamics in RAS-driven cancers. In a balanced state, nutrient supply is sufficient to maintain energy demand (left). Excessive supply of nutrient availability, in the absence of a parallel increase in energy demand, represents a situation in which the energy required to satisfy energy demand is lower than the available energy (middle). A nutrient-deprived condition provokes metabolic inequity (energy supply < energy demand), leading to energetic stress and, ultimately, metabolic vulnerability (right). Nutrient-replete mutant RAS cells utilize rewired metabolism in their favor (middle panel). In the absence of glucose or glutamine, the metabolic vulnerabilities of mutant RAS cells intensify, leading to energetic stress and ultimately to cell death. Interventions that decrease nutrient consumption abolish redox defense and lead the cells to metabolic imbalance. This results in metabolic vulnerability, a potential therapeutic approach for RAS-driven cancer cells. (right panel).
