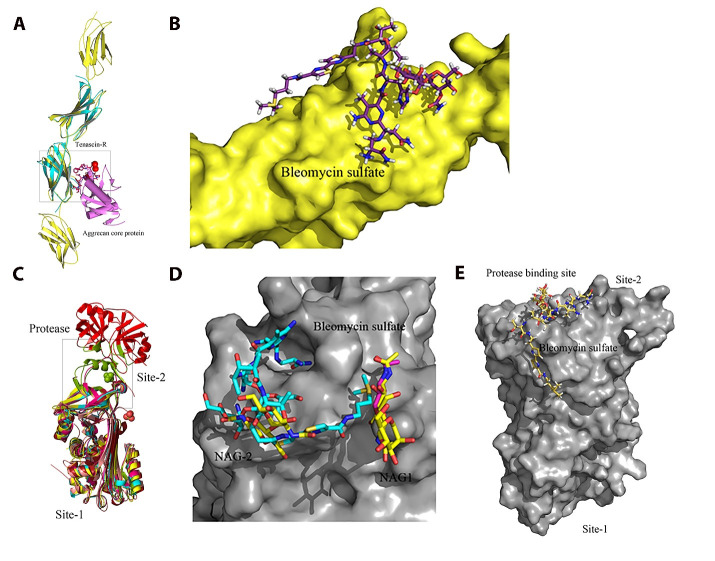
Figure 4. Structural modeling of FN1 (4-domain fragment) and Serpin B5 and docking of bleomycin sulfate, an approved anticancer drug, to FN1 and Serpin B5.
(A). A cartoon representation of the superimposition of the 4-domain fragment of FN1, containing the RGD loop and synergy site, and the complex structure of tenascins and aggrecan lectin domain. (B). Potential binding mode of bleomycin sulfate at the mapped interaction site between FN1 and aggrecan core protein after docking simulations. (C). A carton representation of the superimposition of the crystal structure of Serpin B5 and the crystal structures of its homologues to identify the protease binding sire (Site 2). (D). Potential binding mode of bleomycin sulfate at the N-acetyl-D-glucosamine (NAG) binding site (Site 1) of Serpin B5 after docking simulations. (E). Potential binding mode of bleomycin sulfate at the protease binding site (Site 2) of Serpin B5 after docking simulations.