Fig. 1. Optimized nS/MARt vectors efficiently modify cells in culture and provide prolonged transgene expression in primary CD3+ cells.
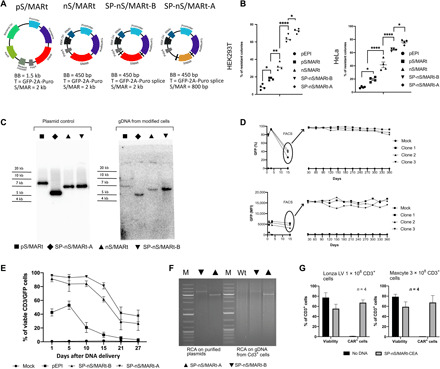
The vectors pS/MARt, nS/MARt, SP-nS/MARt-B, and SP-nS/MARt-A [(A) BB, bacterial backbone; T, transgene] were tested for their efficacy in generating stable clones with a colony-forming assay in HEK293T and HeLa (B). n = 4. In the graph, the line is at the median, and the analysis was performed with a one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for multiple comparisons. *P < 0.05, **P < 0.01, and ****P < 0.0001. The episomal maintenance of the vectors was investigated by Southern blot (C) upon total genomic DNA (gDNA) extraction and digestion with the endonuclease Bam HI. The digested DNA was resolved on an agarose gel, transferred, and hybridized with a radioactive GFP probe. With SP-nS/MARt-EF1a, three independent J76-GFP stable cell lines were generated. The number of viable GFP+ and the medium fluorescent intensity (MFI) of cells were monitored, and 15 days after DNA delivery, GFP+ cells were isolated through FACS and further cultured for 360 days. GFP % and GFP MFI were monitored once per month (D). The GFP-prolonged expression from SP-nS/MARt vectors was investigated in primary human CD3+ cells isolated from four healthy volunteers. The number of CD3/GFP+ cells was monitored at days 1, 5, 10, 15, 21, and 27 after DNA delivery (E), and the episomal maintenance of the vectors was investigated by rolling circle amplification (RCA). The RCA reaction was digested with the endonuclease Bam HI and resolved on a 0.8% agarose gel [(F) M, molecular weight DNA marker; Wt, CD3+ cells unmodified]. SP-nS/MARt vectors expressing the CEA-CAR were optimized to be efficiently delivered with high viability into human CD3+ cells with two large-scale electroporation devices; the Lonza LV Unit and the MaxCyte ExPERT GTx platform (G).
