Fig. 3. In vivo targeted tumor killing.
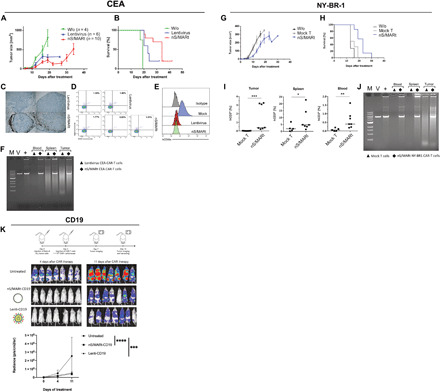
The efficacy of targeting tumor cells expressing the human epitopes CEA, NY-BR1, and CD19 was evaluated in preclinical models. For the CEA model, 1 × 106 cells were implanted subcutaneously in NSG mice, and on day 7, the mice were treated with mock, and CAR-T cells were generated with lentivirus and nS/MARt. Tumor growth (A) and mice survival (B) were monitored. nS/MARt CAR-T cells showed a higher tumor infiltration when compared to Lenti CAR-T cells (C), while the human T cells engraftment in both groups appeared similar (D). The outgrown tumors showed an antigen loss in the treated groups, while it remains expressed in the control group (E). CAR-T cells from the spleens, tumors, and total blood of the treated mice were isolated, and an RCA demonstrated the presence of the episomal plasmids [(F). M. DNA molecular marker; V, nS/MARt-CEA DNA restriction analysis; +, RCA on purified nS/MARt-CEA subsequentially digested with restriction enzymes]. Similarly, for the NY-BR1 model, cells expressing the epitope were xenografted into NSG mice. The efficacy of tumor killing was recorded as tumor growth (G) and survival (H). CAR-T cells in the tumors, spleens, and blood of treated mice were found 30 days after injection (I), and the episomal maintenance of the vector was demonstrated by RCA (J). Nalm-6 cells (1 × 105) modified to express the luciferase constitutively were injected into NSG mice by tail vein injection. Three days after tumor cell injection, 1 × 106 CD19-CAR-T cells generated with mock, nS/MARt, or lentivirus were administered. The tumor growth was monitored through bioluminescent imaging (K), as the radiance (p/s/cm2/sr), and the statistical analysis was performed with a one-way ANOVA followed by post hoc analysis (**P < 0.001 and ***P < 0.001).
