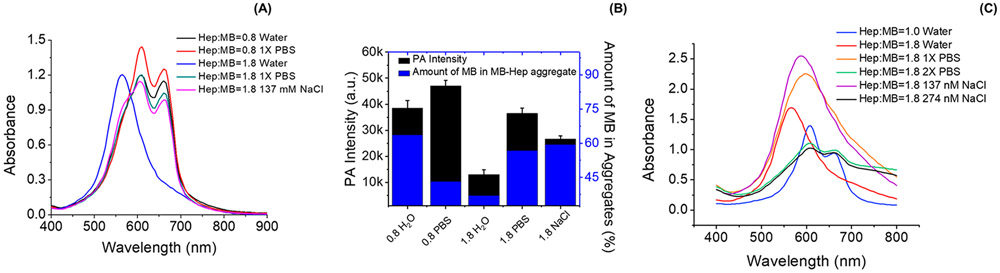
Figure 2.
Impact of PBS in the MB–heparin binding. Panel A compares the spectral change of MB–heparin complex in water and PBS. The MB–heparin sample with heparin:MB at 0.8 in PBS (red) had 24% and 9% higher absorbance at 610 and 660 nm than the sample prepared in water (black). The addition of 1xPBS to a mixture with heparin:MB of 1.8 in water (blue) shifted the absorbance from 570 nm to 610 nm (green). A similar effect was observed upon the addition of 137 mM NaCl (purple) Panel B shows the PA intensity (black) and the amount of MB in the MB–heparin aggregates (blue) of the samples in Panel A. At heparin:MB of 0.8, PBS (0.8 PBS) increased the PA signal of the sample in water (0.8 H2O) by 22%. At heparin:MB of 1.8, adding PBS (1.8 PBS) and 137 mM NaCl (1.8 NaCl) in the sample prepared in water (1.8 H2O) resulted in 2.8- and 2.0-fold increase in PA intensity as well as 1.5- and 1.6-fold in the amount of MB in MB–heparin aggregate. Panel C is the absorbance of 2.4 mM MB with heparin:MB ratio at 1.0 (blue) and 1.8 (red). Adding 1 × PBS (orange) or 137 mM NaCl (purple) only red-shifted the absorbance to 590 and 600 nm. Doubling the PBS (green) or NaCl concentration (black) in the sample could shift the absorbance to 610 nm, like the absorbance of sample with Heparin:MB ratio at 1.0. This indicates that the ionic strength governs the disassociation of MB self-aggregates to MB dimer.