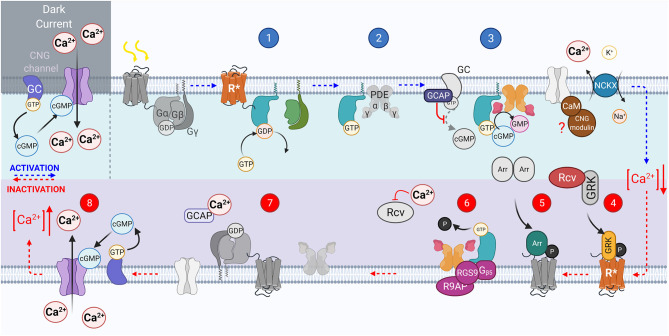
Figure 2.
The vertebrate phototransduction cascade, inactivation, and adaptation mechanisms. The key steps within the vertebrate phototransduction cascade (blue numbering from top left to right) and its subsequent inactivation (red numbering from bottom right to left). In the dark, the CNG channel maintains a dark current, in which Ca2+ ions influx through open CNG channels as the Guanyl Cyclase enzyme is constituently active. Upon absorption of a photon, the chromophore 11-cis-retinal is isomerized to all-trans-retinal, causing the receptor to become activated (R*). In turn, the associated G protein, transducin, is activated with the exchange of GDP for GTP, (step 1). then displaces PDEβγ subunits (step 2). The displaced PDEγ subunit allows cGMP to be reduced to GMP, reducing the concentration of cGMP, closing CNG channels, causing a hyperpolarization of the photoreceptor (step 3). The activated receptor, R*, is inactivated by GRK mediated phosphorylation (step 4). Followed by Arrestin binding (step 5). The GAP protein complex formed of RGS9, R9AP, and Gβ5 bound to both PDEγ and activated causes hydrolysis of GTP on the GTα to GDP (step 6). GTα then dissociates from PDE, reassociating to the βγ subunits, and PDEγ also inhibits the PDEαβ to prevent cGMP hydrolysis (step 7). Intracellular Ca2+ concentration is raised as CNG channels reopen in response to the increasing cGMP concentration, reestablishing the dark current (step 8).