Figure 3.
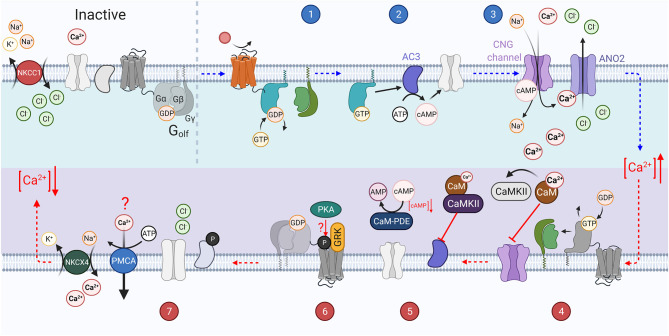
The vertebrate olfactory transduction cascade, inactivation, and adaptation mechanisms: OSNs maintain a high intracellular Cl− ion concentration through the constitutive activity of the NKCC1 transporter, which transports Cl− ions into the cell using the energy from the export of Na+ and K+ ions. An odorant binds to the G-protein coupled olfactory receptor (OR) on the cilium of the sensory neuron. The activated receptor in turn activates Golf (step 1). The Gαolf subunits then activate adenylyl cyclase III (AC3) which catalyzes the conversion of ATP to cAMP (step 2). The increasing concentration of cAMP causes the opening of CNG channels on the membrane. This causes an influx of Ca2+ ions. The increasing Ca2+ concentration also causes the opening of the calcium activated chloride ion channel, ANO2, which further depolarizes the cell as the increased intracellular chloride gradient causes an efflux of Cl−. The increased Ca2+ concentration leads to several inactivation mechanisms (step 3). GTP bound to the active Gαolf subunit is hydrolyzed to GDP, inactivating it, and causing it to re-associate with the βγ subunits. Ca2+ binds to Calmodulin (CaM) which in turn activates CaMKII. Calmodulin potentially reduces CNG channel affinity for cAMP through interaction with the A-subunit of the channel (step 4). CaMKII inhibits the activity of AC3 by phosphorylating it, preventing the generation of cAMP. PDE1C is also activated by Ca2+ bound CaM and accelerates the hydrolysis of cAMP to AMP (step 5). GRK mediated phosphorylation of the OR (step 6). Intracellular Ca2+ concentration is re-established through the activity of ion exchangers (NCKX4 and possibly PMCAs), and the high intracellular Cl− concentration is reestablished through NKCC1 activity and the closure of ANO2 channels (step 7).