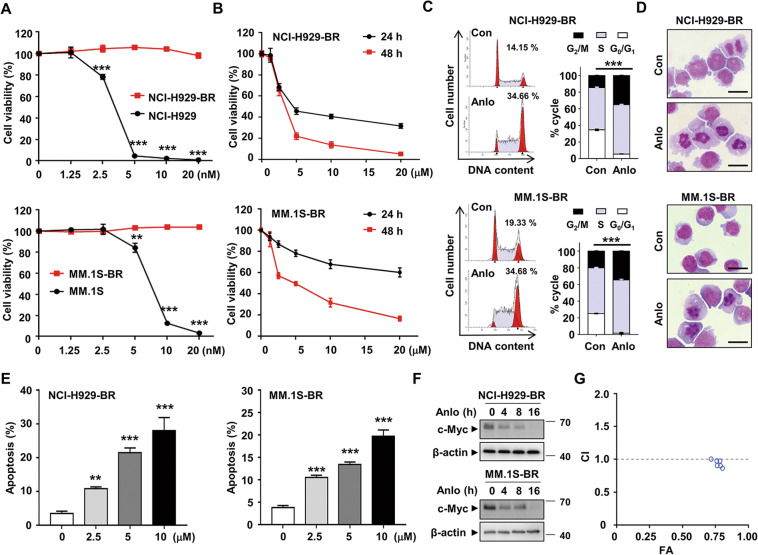
Fig. 6. Anlotinib exhibits cytotoxicity against bortezomib-resistant MM cells.
A NCI-H929, NCI-H929-BR, MM.1S, and MM.1S-BR cells were treated with various concentrations of bortezomib for 24 h. Cell viability was measured by CCK8 assay. B NCI-H929-BR and MM.1S-BR cells were treated with anlotinib (0–20 µM) for 24 and 48 h, followed by an assessment of cell viability. C NCI-H929-BR and MM.1S-BR cells were treated with 5 µM anlotinib for 8 h. The cell cycle was analyzed by flow cytometry. D The morphology of NCI-H929-BR and MM.1S-BR cells after anlotinib treatment (5 μM, 8 h) was observed using Wright staining. E Apoptosis of cells treated with 5 µM anlotinib for 24 h was detected by flow cytometry using Annexin V/PI staining. F NCI-H929-BR and MM.1S-BR cells were treated with anlotinib (5 μM) for the indicated time. Whole cell lysates were subjected to western blotting. G The combined effects of anlotinib and bortezomib in NCI-H929 cells were assessed using the CompuSyn software. Con: control group; Anlo: anlotinib group. ***P < 0.001. Each experiment was performed in triplicate.