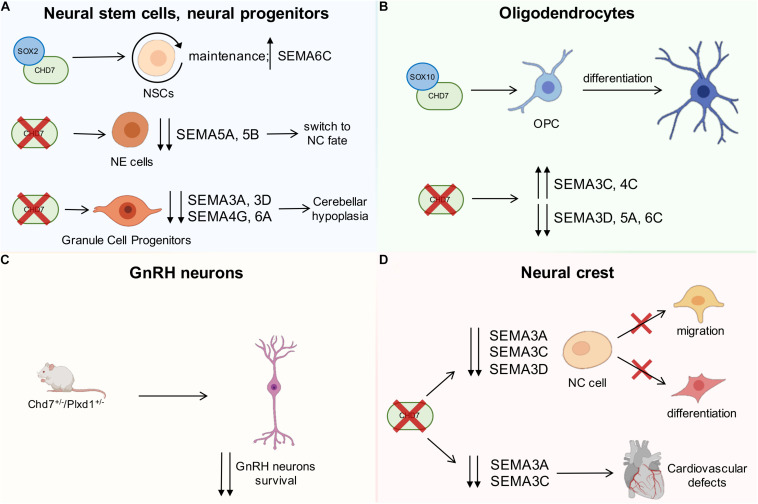
FIGURE 2.
Schematic drawing showing the interactions between CHD7 and semaphorins during development. (A) CHD7 interacts with SOX2 transcriptional cofactor, which is essential for neural stem cell maintenance, and the expression of SEMA6C is directly controlled by CHD7/SOX2 (upper part of the drawing). Insufficient CHD7 activity in neuroepithelial cells leads to downregulation of the indicated semaphorins and switch to a neural crest (NC) fate (middle drawing). In the cerebellum (bottom), CHD7 depletion in granule cell progenitors reduces the expression of the indicated semaphorins and leads to hypoplasia. (B) CHD7 is expressed in the oligodendrocyte lineage of the brain, and CHD7/SOX10 interaction leads to the differentiation of oligodendrocyte precursor cells (OPCs). CHD7 depletion in OPCs induces the upregulation of SEMA3C and 4C and the downregulation of SEMA3D, 5A, and 6C. (C) Compound heterozygous mice for the SEMA3E-receptor PLXND1 and CHD7 display a more significant loss of GnRH neurons compared to single heterozygous mutants. (D) CHD7 is highly expressed in NC pre-migratory cells, and its depletion causes the downregulation of SEMA3A, 3C, and 3D, which are known to regulate cell migration and differentiation. CHD7 is also expressed by a subset of NC cells that participate in the development of the heart. In this context, diminished SEMA3A and SEMA3C expression due to the absence of CHD7 leads to cardiovascular defects. Red crosses indicate a lack of the indicated gene or impairment of the indicated biological process; the up and down arrows indicate expression changes of the indicated genes.