Highlights
-
•
This is the first study about suicide and accident death in patients with bone tumors.
-
•
Patients had higher risk of suicide and accident death than all residents.
-
•
Amputation increased suicidal risk but did not increase the risk of accident death.
-
•
Clinicians should pay more attention to the psychological status in these survivors.
Keywords: Malignant bone tumors, Amputation, Psychological disorders, Suicide
Abstract
Background
It has been recognized that cancer is associated with a higher risk of suicide or accidental death. Earlier studies have evidenced that patients with malignant bone tumors usually experience psychological dysfunction and physical disability following surgery, which are shared risk factors between suicidal and accidental deaths. To our knowledge, there is no large population-based study on the risk of suicide or accidental death among patients with malignant bone tumors.
Questions/purposes
This study aimed to determine whether patients with primary malignant bone tumors are at a higher risk of suicide and accidental death than the general population and to identify the demographic and tumour-related characteristics and type of surgery associated with a higher risk of suicide and accidental death among these patients.
Methods
Overall, 50,817 patients diagnosed with primary malignant bone tumors between 1973 and 1975 were identified from the Surveillance, Epidemiology, and End Results database. The standardised mortality ratio (SMR) was calculated based on the general population’s mortality data, gathered by the National Center for Health Statistics. The Cox regression model was developed to determine risk factors associated with a higher risk of suicide and accidental death.
Results
Patients with primary malignant bone tumors had a higher risk of suicide and accidental death than the general population in the United States (US) (SMR = 2.17; 95% confidence interval (CI) [1.80–2.62] and SMR = 1.73; 95% CI [1.54–1.95]). Compared with limb salvage, amputation significantly increased the risk of suicide (SMR = 3.99; 95% CI [2.52–6.34], hazard ratio (HR) = 2.32; 95% CI [1.31–4.09]; P < 0.01) but did not increase the risk of accidental death (SMR = 1.61; 95% CI [1.07–2.42], HR = 1.11; 95% CI [0.71–1.74]; P = 0.65). Higher suicide risk was observed among older patients whose age at diagnosis was more than 60 years (HR = 4.04; 95% CI [1.98–8.26]; P < 0.001), males (HR = 3.48; 95% CI [2.16–5.62]; P < 0.001), and whites (HR = 3.71; 95% CI [1.17–11.73]; P < 0.001). The risk of suicide and accidental death was highest in the first year after diagnosis (SMR = 2.95; 95% CI [1.86–4.69] and SMR = 2.02; 95% CI [1.48–2.74]).
Conclusion
We first reported that patients with primary malignant bone tumors had a higher risk of suicide and accidental death than the general US population. Therefore, clinicians should pay more attention to the psychological status, physical function, and cognitive level of these survivors.
1. Introduction
Suicide and accidental deaths are a global phenomenon that occurs throughout the lifespan [1]. In 2016, suicide was the 10th leading cause of death in the United States (US) and the second leading cause of death among 15–29 year olds worldwide [2], [3]. An estimated 800,000 people die from suicide every year globally [3]. Accidents were the third leading cause of death in the US, accounting for approximately 6% of all deaths, and it is estimated that 160,000 people die from accidents every year [2]. Cancer is associated with a higher risk of suicide or accidental death [1], [4], and the risk has continued to increase during recent decades [1], [5]. The common contributory factors between suicide and accidental death include psychological illnesses such as depression and anxiety, physical disability, poor quality of life, and social dysfunction [6].
Among patients with malignant bone tumors, changes in gait, function, stability, strength, and appearance have been observed and are related to disease and treatment [7], [8]. The change from being a healthy individual to becoming a disabled person creates a new identity; it takes a long time for patients with bone tumors to accept this new identity [8]. During this process of shift, patients with bone tumors often experience serious psychological distress and problems in social life [9], [10], which has been proven to increase the risk of suicide and accidental death [6], [11], [12], [13]. Furthermore, higher levels of depression and anxiety have been shown in cancer survivors with amputation, especially within the first 2 years after amputation [14], [15].
Stephanie et al. found a relative risk of nearly twice for suicide among cancer patients compared with the general US population [4]. Camidge et al. reported that, in Scottish, the risk of accidental death in cancer patients was 1.58 times that in the general population [16]. However, none of these reports on the risk of suicide or accidental death among cancer patients has isolated bone tumors as a single subgroup. To the best of our knowledge, currently, there is no large population-based study on the risk of suicide or accidental death among patients with malignant bone tumors. Therefore, the objectives of this study were to evaluate the risk of suicide and accidental death among patients with malignant bone tumors relative to that in the general population and to identify the characteristics linked to a higher risk of suicide and accidental death.
2. Patients and methods
This retrospective cohort study identified patients diagnosed with malignant bone tumors between January 1, 1973, and December 31, 2017, from the Surveillance, Epidemiology, and End Results (SEER) database established by the National Cancer Institute, which covered approximately 28% of the US population [17]. For comparison, the mortality data of the general US population collected by the National Center for Health Statistics spanning from 1969 to 2017 was used.
To exclude the impact of a secondary tumour on suicide or accidental death, we only included patients diagnosed with the first primary malignant bone tumors in this study, and those who had no age at diagnosis and survival time data were excluded. All patients were divided into seven subgroups according to the primary site of bone tumour, including the upper limb, lower limb, skull and face, vertebral column, thorax, pelvis, and others (specific primary site codes are listed in Table 1).
Table 1.
The risks of suicide and accident death among patients with primary malignant bone tumors diagnosed between 1 January 1973 and 31 December 2017.
| Variable | No. of Patients | Person-years | Suicides† |
Accident deaths† |
||||
|---|---|---|---|---|---|---|---|---|
| No. of deaths | Mortality rate‡ | SMR (95%CI) | No. of deaths | Mortality rate‡ | SMR (95%CI) | |||
| Sex | ||||||||
| Female | 22,039 | 182281.96 | 21 | 11.52 | 2.88 (1.88–4.42) | 82 | 44.99 | 2.64 (2.12–3.27) |
| Male | 28,778 | 214509.54 | 87 | 40.56 | 2.74 (2.22–3.38) | 184 | 85.78 | 2.26 (1.95–2.61) |
| Race | ||||||||
| Black | 4874 | 37689.46 | 3 | 7.96 | 1.80 (0.58–5.57) | 17 | 45.11 | 1.81 (1.13–2.92) |
| White | 41,682 | 326542.33 | 105 | 32.16 | 2.97 (2.45–3.60) | 232 | 71.05 | 2.39 (2.10–2.72) |
| Other | 4261 | 32559.71 | 0 | 0.00 | 0.00 | 17 | 52.21 | 2.75 (1.71–4.42) |
| Year of diagnosis | ||||||||
| 1973–1984 | 5275 | 73548.88 | 29 | 39.43 | 3.07 (2.13–4.42) | 38 | 51.67 | 1.42 (1.03–1.95) |
| 1985–1995 | 7091 | 95095.42 | 18 | 18.93 | 1.84 (1.16–2.91) | 53 | 55.73 | 1.95 (1.49–2.55) |
| 1996–2006 | 17,281 | 153892.67 | 35 | 22.74 | 2.59 (1.86–3.61) | 120 | 77.98 | 3.05 (2.55–3.64) |
| 2007–2017 | 21,170 | 74254.54 | 26 | 35.01 | 4.14 (2.82–6.08) | 55 | 74.07 | 2.87 (2.20–3.74) |
| Income | ||||||||
| High | 18,538 | 151906.83 | 25 | 16.46 | 2.38 (1.61–3.53) | 96 | 63.20 | 3.71 (3.04–4.53) |
| Median | 16,450 | 119125.50 | 34 | 28.54 | 2.98 (2.13–4.17) | 75 | 62.96 | 2.53 (2.01–3.17) |
| Low | 13,269 | 89292.67 | 34 | 38.08 | 2.67 (1.91–3.74) | 73 | 81.75 | 1.77 (1.41–2.23) |
| Unknown | 2560 | 36466.50 | 15 | 41.13 | 3.38 (2.04–5.61) | 22 | 60.33 | 1.39 (0.92–2.12) |
| Education | ||||||||
| High | 10,241 | 59888.38 | 18 | 30.06 | 2.16 (1.36–3.44) | 58 | 96.85 | 2.03 (1.57–2.63) |
| Median | 11,361 | 92900.33 | 24 | 25.83 | 1.95 (1.31–2.91) | 42 | 45.21 | 1.22 (0.90–1.65) |
| Low | 11,627 | 103928.04 | 29 | 27.90 | 2.07 (1.44–2.98) | 74 | 71.20 | 2.19 (1.74–2.74) |
| Unknown | 17,588 | 140074.75 | 37 | 26.41 | 8.34 (6.04–11.51) | 92 | 65.68 | 5.83 (4.75–7.15) |
| Stage | ||||||||
| Localized | 18,189 | 181461.88 | 48 | 26.45 | 2.60 (1.96–3.45) | 124 | 68.33 | 2.40 (2.01–2.86) |
| Regional | 17,340 | 144462.67 | 47 | 32.53 | 3.35 (2.51–4.45) | 94 | 65.07 | 2.30 (1.88–2.81) |
| Distant | 8628 | 31543.33 | 4 | 12.68 | 1.47 (0.55–3.92) | 18 | 57.06 | 2.21 (1.39–3.51) |
| Unknown | 6660 | 39323.63 | 9 | 22.89 | 2.37 (1.23–4.55) | 30 | 76.29 | 2.53 (1.77–3.61) |
| Site§ | ||||||||
| Lower limb | 20,774 | 184135.63 | 55 | 29.87 | 3.46 (2.65–4.50) | 101 | 54.85 | 2.18 (1.80–2.65) |
| Upper limb | 6409 | 55450.29 | 16 | 28.85 | 2.95 (1.81–4.82) | 26 | 46.89 | 1.67 (1.14–2.45) |
| Pelvis | 7930 | 45587.33 | 15 | 32.90 | 2.74 (1.65–4.55) | 39 | 85.55 | 2.54 (1.86–3.48) |
| Skull and Face | 6910 | 52090.17 | 13 | 24.96 | 2.34 (1.36–4.04) | 58 | 111.35 | 3.57 (2.76–4.62) |
| Thorax | 3736 | 30705.58 | 6 | 19.54 | 1.67 (0.75–3.72) | 6 | 19.54 | 0.59 (0.26–1.31) |
| Vertebral column | 3507 | 22272.33 | 3 | 13.47 | 1.21 (0.39–3.76) | 33 | 148.17 | 4.83 (3.43–6.79) |
| Others | 1551 | 6550.17 | 0 | 0.00 | 0.00 | 3 | 45.80 | 1.40 (0.45–4.35) |
| Type¶ | ||||||||
| Osteosarcoma | 17,155 | 133144.29 | 46 | 34.55 | 4.61 (3.45–6.15) | 76 | 57.08 | 2.53 (2.02–3.17) |
| Chondrosarcoma | 13,895 | 126683.83 | 39 | 30.79 | 2.41 (1.76–3.29) | 69 | 54.47 | 1.56 (1.23–1.98) |
| Ewing sarcoma | 7294 | 53812.63 | 10 | 18.58 | 3.04 (1.63–5.65) | 25 | 46.46 | 2.49 (1.68–3.68) |
| Chordoma | 3982 | 26073.29 | 3 | 11.51 | 0.86 (0.28–2.68) | 38 | 145.74 | 3.96 (2.88–5.45) |
| Others | 8491 | 57077.46 | 10 | 17.52 | 1.64 (0.88–3.06) | 58 | 101.62 | 3.09 (2.39–3.99) |
| Surgery | ||||||||
| Yes | 38,116 | 329260.58 | 85 | 25.82 | 2.57 (2.08–3.18) | 207 | 62.86814 | 2.23 (1.94–2.55) |
| None | 10,108 | 46175.50 | 10 | 21.66 | 2.61 (1.41–4.86) | 47 | 101.79 | 3.71 (2.79–4.94) |
| Unknown | 2593 | 21355.42 | 13 | 60.87 | 6.11 (3.55–10.53) | 12 | 56.19 | 1.70 (0.97–3.00) |
| All | 50,817 | 396791.50 | 108 | 27.22 | 2.77 (2.29–3.34) | 266 | 67.04 | 2.36 (2.09–2.66) |
Abbreviations: SMR, standardized mortality ratio; CI, confidence intervals.
In this study, we included all patients with primary malignant bone tumors reported to the eighteen SEER registries (Alaska Native Tumor registry, Connecticut, Detroit, Atlanta, Greater Georgia, Rural Georgia, San Francisco–Oakland, San Jose-Monterey, Greater California, Kentucky, Los Angeles, Louisiana, Hawaii, Iowa, New Mexico, New Jersey, Seattle–Puget Sound, and Utah) diagnosed between January 1, 1973, and December 31, 2017. The data contributed by Idaho, Massachusetts and New York from January 1, 2000, and December 31, 2017 were also available.
Suicide: International Classification of Diseases, Eighth Revision codes 950–959; International Classification of Diseases, Ninth Revision codes 950–959; and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes U03, X60-X84 and Y87.0. Accident death: International Classification of Diseases, Eighth Revision codes 800–949; International Classification of Diseases, Ninth Revision codes 800–949; and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes V01-X59 and Y85-Y86.
Per 100,000 person-years.
Specific primary site code (the International Statistical Classification of Diseases and Related Health Problems, the tenth revision [ICD-10 codes]): skull and face: C41.0, C41.1; thorax: C41.3; vertebral column: C41.2; pelvis: C41.4; upper limb: C40.0, C40.1; lower limb: C40.2, C40.3; others: C40.8, C40.9, C41.8, C41.9.
The histological codes (International Classification of Diseases for Oncology, 3rd Edition [ICD-O-3]): Osteosarcoma: 9180/3–9200/3; Chondrosarcoma: 9220/3–9243/3; Ewing sarcoma: 9260/3; Chordoma: 9370/3–9372/3; Others: 8000/3–9150/3, 9250/3–9251/3, 9261/3–9365/3 and 9380/3–9580/3.
We extracted the demographic characteristics from the SEER database comprising age at diagnosis (0–14, 15–29, 30–44, 45–59, and 60 + ), sex (female, male), race (white, black, and other), and calendar year of diagnosis (1973–1984, 1985–1995, 1996–2006, and 2007–2017). The tumour-related variables comprised the primary site and clinical stage of bone tumors (local, regional, distant, and unknown). All bone tumors were classified according to the International Classification of Disease for Oncology third revision (ICD-O-3) into five subtypes, including osteosarcoma, chondrosarcoma, Ewing sarcoma, chordoma, and others (ICD-O-3 codes are listed in Table 1). The surgery of bone tumors was categorised into “amputation”, “limb salvage”, “surgery, not otherwise specified (NOS)”, “none”, and “unknown”. Survival time and cause of death were also available.
Patients with a cause of death coded as “Suicide and Self-Inflicted Injury (50220)” were considered to have committed suicide, and those with a cause of death coded as “Accidents and Adverse Effects (50210)” were considered to have died from accidents .
The number of suicides or accidental deaths divided by person-years of survival was calculated as the rate of suicide or accidental death. The data of patients with bone tumors were compared with those of the general population with similar distributions of age, sex, and race. Among cancer patient subgroups stratified by different characteristics, the standardised mortality ratios (SMRs) and 95% confidence intervals (95% CIs) were computed as described earlier [18]. Five-year age ranges were used for standardisation. To determine the demographic and tumour-related characteristics and type of surgery that was associated with a higher risk of suicide and accidental death, we developed a multivariate Cox proportional hazards model. Observations were censored if patients did not die from suicide or accidents at the time of the last follow-up. Survival time recorded as 0 months in the SEER database was converted to one-half of a month according to accepted epidemiologic practices [18].
All statistical tests were two-sided, and the values with P < 0.05 were considered statistically significant. The analysis was performed using the SEER*Stat software version 8.3.6 and the R version 3.51 statistical software.
3. Results
The analysed data revealed that a total of 108 suicides and 266 accidental deaths occurred among 50,817 patients with bone tumors, followed by 396,791 person-years. The suicide rate was 27.22/100,000 person-years, and the rate of accidental death was 67.04/100,000 person-years among cancer patients (Table 1). Patients with primary malignant bone tumors had a significantly higher risk of suicide and accidental death than the general US population with the same distribution of age, sex, and race (SMR = 2.17; 95% CI [1.80–2.62] and SMR = 1.73; 95% CI [1.54–1.95]). The survival time ranged from 0 to 42.92 years, with a mean survival time of 7.80 years.
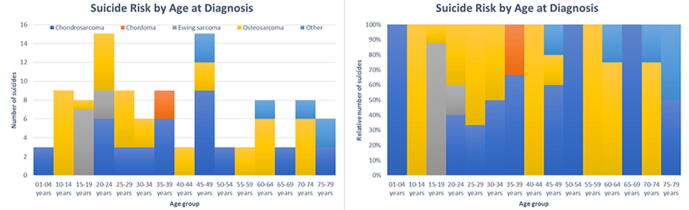
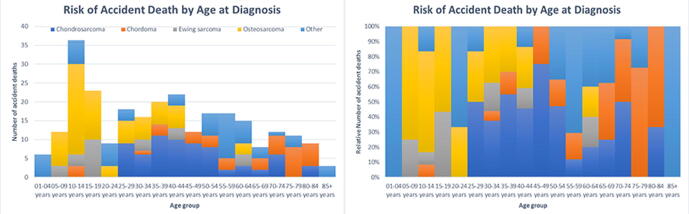
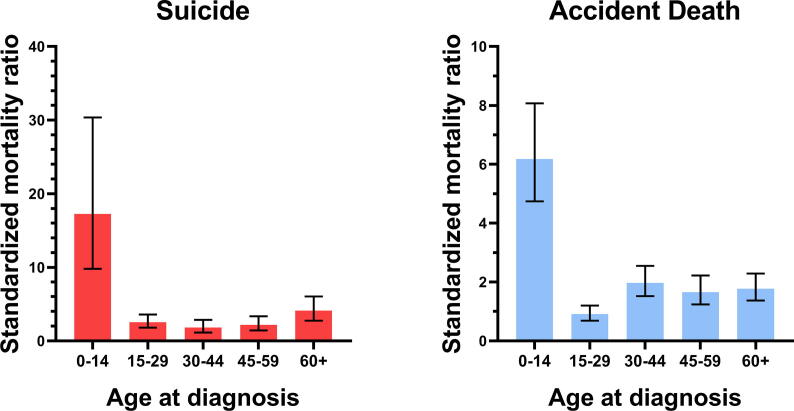
3.1. Subgroups of patients with malignant bone tumors related to a higher risk of suicide and accidental death
There were 12 suicides and 54 accidental deaths observed among patients with malignant bone tumors whose age at diagnosis was less than 15 years old Fig. 1 and 2. These children with bone tumors had a higher risk of suicide and accidental death than the matched general population (SMR = 16.54; 95% CI [9.39–29.12] and SMR = 6.18; 95% CI [4.74–8.07]) (Figure 3). The old patients had a higher risk of suicide and accidental death among all age subgroups (SMR = 2.84; 95% CI [1.92–4.21] and SMR = 1.77; 95% CI [1.37–2.29]). Males had a higher risk of suicide and accidental death than the females (SMR = 2.17; 95% CI [1.76–2.67] and SMR = 2.19; 95% CI [1.43–3.36]). Patients with regional bone tumors and those with localised bone tumors were both equally likely to die from suicide (SMR = 2.61; 95% CI [1.96–3.48] and SMR = 2.06; 95% CI [1.55–2.74]) and accidents (SMR = 1.68; 95% CI [1.37–2.06] and SMR = 1.79; 95% CI [1.50–2.14]). The risk of suicide and accidental death for patients with advanced bone tumors was equal to that of the general population (SMR = 1.12; 95% CI [0.42–2.97] and SMR = 1.47; 95% CI [0.92–2.33]).
Fig. 1.
Standardized mortality ratios (SMRs) of suicide among patients with malignant bone tumors by age at diagnosis.
Fig. 2.
The y-axis represented the absolute or relative number of suicides and the x-axis represented the age group at diagnosis. The colors depicted the histological subtypes of malignant bone tumors.
Fig. 3.
The y-axis represented the absolute or relative number of accident deaths and the x-axis represented the age group at diagnosis. The colors depicted the histological subtypes of malignant bone tumors.
3.2. Sites of malignant bone tumors associated with a higher risk of suicide and accidental death
The suicide risk was the highest in patients with malignant bone tumors of the pelvic (32.90/100,000 person-years; SMR = 2.11; 95% CI [1.27–3.50]), followed by those with lower limb bone tumors (29.87/100,000 person-years; SMR = 2.79; 95% CI [2.14–3.64]) and upper limb (28.85/100,000 person-years; SMR = 2.35; 95% CI [1.44–3.83]). The highest risk of accidental death was observed in patients with bone tumors of the vertebral column (148.17/100,000 person-years; SMR = 3.50; 95% CI [2.49–4.93]), followed by those with bone tumors of the skull and face (111.35/100,000 person-years; SMR = 2.66; 95% CI [2.05–3.44]) and pelvic (85.55/100,000 person-years; SMR = 1.88; 95% CI [1.37–2.57]).
3.3. Pathological types of malignant bone tumors associated with a higher risk of suicide and accidental death
Among patients with osteosarcoma, there were 46 suicides of 17,155 patients, accounting for 35% of all suicides among patients with malignant bone tumors. The patients with osteosarcoma had the highest suicide risk among all patients with bone tumors (34.55/100,000 person-years; SMR = 3.72; 95% CI [2.79–4.97]). The patients with chordoma had a minimal number of suicides, and those had the highest risk of accidental death (145.74/100,000 person-years; SMR = 2.98; 95% CI [2.17–4.09]).
3.4. Types of surgery related to a higher risk of suicide and accidental death
A total of 85 suicides and 207 accidental deaths were identified among patients who underwent surgery for malignant bone tumors. Compared with patients who did not undergo surgery (SMR = 1.84; 95% CI [0.99–3.43]), the patients who underwent surgery (SMR = 2.02; 95% CI [1.63–2.49]) had a higher suicide risk. Among patients who underwent surgery, amputation had a significantly higher suicide risk than those who underwent limb salvage surgery (SMR = 3.99; 95% CI [2.52–6.34] and SMR = 1.53; 95% CI [1.12–2.07]) (Table 2). Patients who did not undergo surgery had a higher risk of accidental death than those who underwent surgery (SMR = 2.42; 95% CI [1.82–3.22] and SMR = 1.64; 95% CI [1.43–1.88]). Among patients who underwent surgery, patients who underwent amputation or limb salvage had a higher risk of accidental death compared with the general population (SMR = 1.61; 95% CI [1.07–2.42] and SMR = 1.67; 95% CI [1.41–1.98]).
Table 2.
The risks of suicide and accident death among patients who underwent different surgery for malignant bone tumors.
| Surgery | No. of Patients | Suicides |
Accident deaths |
||||
|---|---|---|---|---|---|---|---|
| No. of deaths | Mortality rate† | SMR (95%CI) | No. of deaths | Mortality rate† | SMR (95%CI) | ||
| Amputation | 5324 | 18 | 50.05 | 5.47 (3.45–8.68) | 23 | 63.95 | 2.40 (1.60–3.61) |
| Limb Salvage | 27,062 | 41 | 19.58 | 2.18 (1.60–2.96) | 134 | 64.00 | 2.53 (2.14–3.00) |
| Surgery, NOS | 5730 | 26 | 30.99 | 2.37 (1.61–3.48) | 50 | 59.59 | 1.64 (1.25–2.17) |
Abbreviations: SMR, standardized mortality ratio; CI, confidence intervals.
Per 100,000 person-years.
3.5. Internal comparisons: risk factors linked to a higher risk of suicide and accidental death based on Cox regression model
Male sex was found to be strongly associated with suicide (HR = 3.48; 95% CI [2.16–5.62]; P < 0.001) and accidental death (HR = 1.99; 95% CI [1.53–2.58]; P < 0.001) among patients with primary malignant bone tumors (Table 3). The risk of suicide for whites was three times that of blacks (HR = 3.71; 95% CI [1.17–11.73]; P = 0.025). Patients with bone tumors of the extremities (HR = 1.66; 95% CI [1.03–2.69]; P = 0.038) or regional bone tumors (HR = 3.07; 95% CI [1.09–8.64]; P = 0.034) had higher suicide risk. Besides, the patients whose age at diagnosis was more than 60 years old were at higher risk of suicide (HR = 4.04; 95% CI [1.98–8.26]; P < 0.001) and accidental death (HR = 1.60; 95% CI [1.09–2.37]; P < 0.001) than other age subgroups. Compared with limb salvage, amputation significantly increased the risk of suicide (HR = 2.32; 95% CI [1.31–4.09]; P < 0.001), Fig. 4 but did not increase the risk of accidental death (HR = 1.11; 95% CI [0.71–1.74]; P = 0.65) Fig. 5, using the Cox proportional hazards model.
Table 3.
Cox regression analyses of suicide and accidental death among patients with malignant bone tumors.
| Variable | Suicides |
Accident deaths |
||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | |
| Age at diagnosis | ||||||
| 0–14 | – | – | Ref | – | – | Ref |
| 15–29 | 1.79 | (0.92, 3.49) | 0.086 | 0.58 | (0.40, 0.86) | 0.006 |
| 30–44 | 1.90 | (0.91, 4.00) | 0.089 | 1.03 | (0.70, 1.51) | 0.873 |
| 45–59 | 2.50 | (1.21, 5.17) | 0.014 | 0.93 | (0.62, 1.39) | 0.718 |
| 60+ | 3.96 | (1.94, 8.10) | 0.000 | 1.61 | (1.09, 2.38) | 0.016 |
| Sex | ||||||
| Female | – | – | Ref | – | – | Ref |
| Male | 3.49 | (2.16, 5.64) | 0.000 | 1.99 | (1.53, 2.59) | 0.000 |
| Race | ||||||
| Black | – | – | Ref | – | – | Ref |
| White | 4.08 | (1.28, 12.94) | 0.017 | 1.42 | (0.86, 2.34) | 0.168 |
| Other | 0.00 | NA | 0.940 | 1.06 | (0.54, 2.09) | 0.865 |
| Year of diagnosis | ||||||
| 1973–1984 | – | – | Ref | – | – | Ref |
| 1985–1995 | 0.77 | (0.36, 1.66) | 0.503 | 1.23 | (0.71, 2.12) | 0.456 |
| 1996–2006 | 1.26 | (0.51, 3.10) | 0.617 | 2.21 | (1.20, 4.08) | 0.011 |
| 2007–2017 | 1.59 | (0.61, 4.14) | 0.346 | 1.91 | (0.98, 3.74) | 0.058 |
| Income | ||||||
| High | – | – | Ref | – | – | Ref |
| Median | 1.57 | (0.94, 2.64) | 0.087 | 0.99 | (0.73, 1.34) | 0.930 |
| Low | 2.60 | (1.42, 4.76) | 0.002 | 1.20 | (0.81, 1.77) | 0.376 |
| Unknown | 2.13 | (0.91, 4.97) | 0.081 | 1.17 | (0.65, 2.10) | 0.593 |
| Education | ||||||
| High | – | – | Ref | – | – | Ref |
| Median | 1.21 | (0.62, 2.37) | 0.580 | 0.56 | (0.36, 0.87) | 0.011 |
| Low | 1.56 | (0.76, 3.22) | 0.230 | 0.93 | (0.59, 1.45) | 0.736 |
| Unknown | 1.32 | (0.64, 2.75) | 0.452 | 0.79 | (0.51, 1.23) | 0.299 |
| Location† | ||||||
| Axial | – | – | Ref | – | – | Ref |
| Extremities | 1.63 | (1.01, 2.64) | 0.047 | 0.77 | (0.57, 1.03) | 0.082 |
| Others | 1.23 | (0.62, 2.44) | 0.551 | 1.40 | (1.00, 1.97) | 0.052 |
| Stage | ||||||
| Distant | – | – | Ref | – | – | Ref |
| Localized | 2.55 | (0.90, 7.22) | 0.077 | 1.53 | (0.92, 2.55) | 0.103 |
| Regional | 3.10 | (1.10, 8.73) | 0.032 | 1.33 | (0.79, 2.22) | 0.281 |
| Unknown | 1.84 | (0.56, 6.05) | 0.317 | 1.32 | (0.73, 2.39) | 0.365 |
| Surgery | ||||||
| Limb Salvage | – | – | Ref | – | – | Ref |
| Amputation | 2.27 | (1.28, 4.01) | 0.005 | 1.11 | (0.71, 1.75) | 0.638 |
| None | 1.34 | (0.66, 2.72) | 0.424 | 1.62 | (1.14, 2.31) | 0.007 |
| Surgery, NOS | 1.65 | (0.74, 3.66) | 0.221 | 1.34 | (0.82, 2.18) | 0.241 |
| Unknown | 4.20 | (1.71, 10.30) | 0.002 | 1.41 | (0.70, 2.86) | 0.335 |
Axial included “Thorax”, “Vertebral column” and “Pelvis”; Extremities included “Lower limb” and “Upper limb”; Others included “Others” and “Skull and Face”.
Fig. 4.
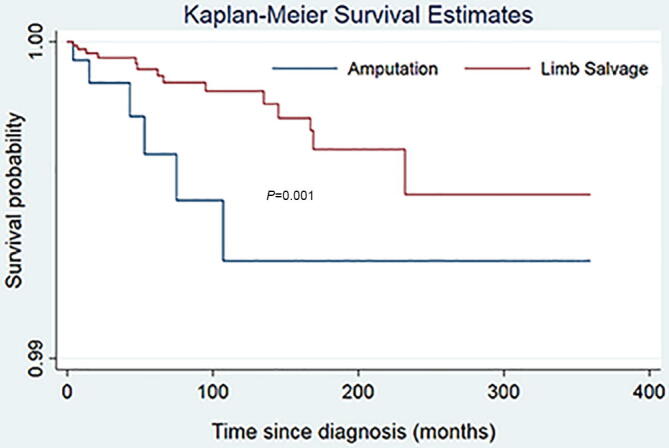
Kaplan-Meier survival curve for suicide among patients with malignant bone tumors. The result of log-rank indicated that there was significant difference of risk of suicide between patients who underwent amputation and those who underwent limb salvage.
Fig. 5.
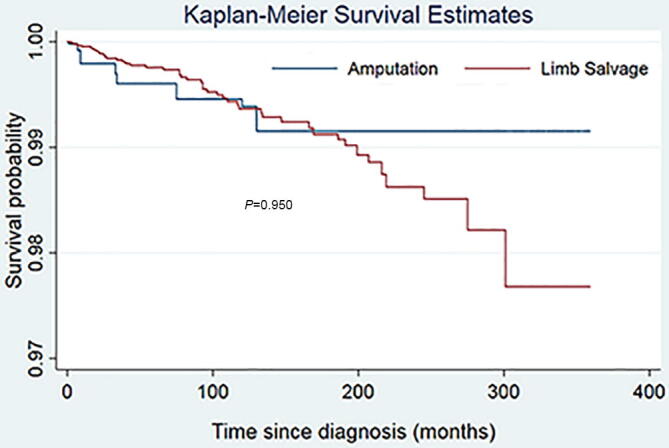
Kaplan-Meier survival curve for accident death among patients with malignant bone tumors. The result of log-rank indicated that there was no difference of risk of suicide between patients who underwent amputation and those who underwent limb salvage.
3.6. Risk of suicide and accidental deaths with time since diagnosis
Among patients with malignant bone tumors, the risk of suicide was the highest in the first year after diagnosis, decreased gradually from 1 year to 10 years, and increased after 10 years Table 4. The risk of accidental death was also the highest in the first year after diagnosis, but decreased gradually from 1 year to 5 years, and increased after 5 years Table 4.
Table 4.
The risk of suicide and accident death by years since diagnosis.
| Time since diagnosis | Suicides |
Accident deaths |
||
|---|---|---|---|---|
| No. of deaths | SMR (95%CI) | No. of deaths | SMR (95%CI) | |
| 0 to 1y | 18 | 4.26 (2.68–6.76) | 41 | 3.04 (2.24–4.13) |
| 1y to 5y | 39 | 2.76 (2.02–3.78) | 75 | 1.80 (1.43–2.25) |
| 5y to 10y | 15 | 0.93 (0.56–1.54) | 61 | 1.34 (1.04–1.72) |
| greater than10y | 36 | 2.71 (1.98–3.75) | 89 | 2.43 (1.98–3.00) |
Abbreviations: SMR, standardized mortality ratio; CI, confidence intervals.
4. Discussion
It has been recognised that cancer patients have a higher risk of suicide or accidental death than the general population [4], [11]. We first reported that the risk of suicide among patients with malignant bone tumors was 2 times that of the general population, and the risk of accidental death was 1.7 times that of the general population. Bone tumors can cause psychological stress, physical disabilities, and social dysfunction [9], [10], which increases the risk of suicide and accidental death [6].
Our study has several limitations. First, the SEER database contains detailed information regarding chemoradiotherapy. Earlier studies have shown that radiotherapy could induce cognitive dysfunction among patients with cancer of the head and neck [19], [20], which might increase the risk of accidental death [11]. Furthermore, we do not know the regimens of radiotherapy from the SEER database, such as cycles, which renders it difficult to assess the direct association between radiotherapy and the risk of accidental death among patients with bone tumors of the skull and face. Second, data on psychiatric status, quality of life, and social support are also unavailable in the SEER database. Therefore, we could not evaluate the impact of these factors on the risk of suicide and accidental death. This study is the first large population-based study on the risk of suicide and accidental death in patients with malignant bone tumors. Hence, the results of the study are reliable and applicable to the rest of the population (Table 4).
Using data from the SEER database, we found that surgery, especially amputation, significantly increased suicide risk among patients with malignant bone tumors. The principle treatment for bone tumors was surgery. Visible changes in the body, such as physical disability and deviating appearance, occurred in patients with bone tumors following surgical treatment [6], [8]. These changes decreased the quality of life and caused a series of mental illnesses such as depression or anxiety [9], [10]. Bone tumors in the pelvis and extremities may require extensive surgery [21], and higher suicide risk in patients with malignant bone tumors of the extremities and pelvis may be related to surgery. It was difficult for patients with bone tumors of the extremities to cover up the physical disabilities and appearance deviation caused by surgery. For these cancer survivors, exposing the deviating appearance to others was a challenge in social situations, which decreased their participation in social and recreational activities and increased the level of depression and demoralization [8]. Higher levels of depression and anxiety have been shown in cancer patients with amputation [14], [15]. The bone tumors of the pelvis were associated with more complicated resections and higher recurrence rates [22], and worse prognosis had a negative effect on psychological healthy [22], [23], [24]. Brianna et al. reported that patients with bone and soft tissue cancer of the pelvis had a higher incidence of suicide [24], which was consistent with our study.
Osteosarcoma is the most common primary malignant bone tumour, and its treatment includes systemic multiagent chemotherapy and complete surgical resection [25]. In our study, the higher suicide risk in patients with osteosarcoma may be associated with surgery and location of osteosarcoma, which is usually found at the end of long bones and around the knees. Limb salvage surgery for osteosarcoma continued to improve with the development of chemotherapy and imaging, which was beneficial to improve the prognosis and might reduce the risk of suicide for patients with osteosarcoma.
The patients with bone tumors of the vertebral column or with chordoma had the highest risk of accidental death among all patients with malignant bone tumors. The higher risk of accidental death among patients with malignant bone tumors may be due to psychological stress, physical disabilities, and poor quality of life [6], [9], [10], [11]. In addition, the tumors themselves and complications played an important role. Patients with spinal tumors usually present with spinal pain, limb weakness, and radiculopathy [26]. The clinical presentations of patients with chordoma mainly depended on the sites of chordoma, which can arise anywhere along the central neural axis from the skull base to the sacrum. Patients with chordomas of the skull base most often presented with headache, diplopia, and visual loss, and patients with spinal chordomas usually present with local deep pain, weakness, and numbness [27], [28]. The symptoms could interfere with the normal work and life of cancer patients [29], [30], might reduce the ability of patients to respond to emergency and increase the risk of death from unintentional injury. The reasons for the association of malignant bone tumors with a higher risk of accidental death warrant further investigation.
Meanwhile, the risk of accidental death in patients with malignant bone tumors of the skull and face was approximately three times that of the general population, and possible hypotheses to explain this association included cognitive impairment and epilepsy. Cancer-related and treatment-induced cognitive dysfunction and epilepsy were common complications among patients with tumors of head and neck, also associated with pre-existing comorbidities [19], [20], [31]. Surgery could improve the complications, thus reducing the risk of accidental death among patients with bone tumors.
Although suicide was the second leading cause of death among the general population aged between 15 and 29 worldwide [3], it was reported that the suicide rate of adolescents and young cancer patients whose age at diagnosis was between 15 and 39 was marginally lower than that of all-age cancer patients [32], which was consistent with the results of our study. We first found that patients with bone tumors whose age at diagnosis was less than 15 had the highest SMR of suicide among all-age cancer patients, and the patients whose age at diagnosis was more than 60 years had a higher suicide risk than other age subgroups. The children and old patients with bone tumors were more likely to make unwise decisions because they could not get enough information about the tumour and psychological support from the Internet [33], [34], [35]. Suicides were preventable, and there were more than 20 attempts per suicide [3]. The Distress Assessment and Response Tool has been used to identify suicidal ideation in patients with cancer [36], and item 9 of the Patient Health Questionnaire depression module (PHQ9) was also a strong predictor of suicide attempts and suicide deaths in patients [37]. It was necessary to select patients whose age at diagnosis was less than 14 or more than 60 out when considering the suicide risk of patients with malignant bone tumors. Moreover, we should enhance these special patients’ understanding of tumors and confidence to defeat diseases.
Our study demonstrated that patients with advanced bone tumors had the same risk of suicide and accidental death as the general population, which was at variance with prior works [4], [11]. Possible hypotheses to explain this was that patients with advanced tumors had a worse prognosis, most of them died from bone tumour shortly after diagnosis, and shorter survival time obscured the risk of suicide or accidental death [38]. White patients with malignant bone tumors had a significantly higher suicide risk than black patients, which could be attributable to economic status, educational level, and culture factors [39], [40].
We also found that the risk of suicide and accidental death was highest within the first year after initial diagnosis among patients with malignant bone tumors. The change in social status from being a healthy individual to being a cancer patient, even a disabled person, created a new identity [8]. Survivors with bone tumors needed enough time to accept these identity changes [8], which resulted in the highest risk of suicide and accidental death shortly after diagnosis [6], [9], [10]. This finding also underlines the concept that to better guide bone cancer survivors during follow-up, healthcare providers should have a comprehensive understanding of what this modification of identity means to survivors with malignant bone tumors [8].
The outcomes of this study indicated that patients with primary malignant bone tumors had a higher risk of suicide and accidental death than the general population, which was related to psychological stress and social dysfunction. It is worth noting that amputation due to bone tumors increased the risk of suicide but did not increase the risk of accidental death. Therefore, when selecting a surgical method for patients with malignant bone tumors, orthopaedic surgeons should not only consider the effect of surgery but also consider the acceptability of patients for postoperative physical changes such as deviating appearance. The suicides are preventable, but preventive measures for accidental deaths need further research. In order to reduce the risk of suicide and accidental death among patients with malignant bone tumors, clinicians should pay more attention to the psychological status, physical function, and cognitive level of these survivors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
None.
Author contributions
K.Y., B.W and F.L. designed research.
K.Y., B.W., and Y.C. calculated data.
K.Y., H.K., K.S., Y.D., and P.P. analyzed result.
K.Y., B.W., Y.C., H.K., K.S., Y.D., P.P., and F.L. wrote this paper.
All authors revised the final version.
Studies in humans and animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data analyzed in this study were from the Surveillance, Epidemiology and End Results database, which was publicly available datasets.
Conflict of Interest
Each author certifies that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Tichelli A., Labopin M., Rovo A., Badoglio M., Arat M., van Lint M.T., Lawitschka A., Schwarze C.P., Passweg J., Socie G. Increase of suicide and accidental death after hematopoietic stem cell transplantation: a cohort study on behalf of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Cancer. 2013;119(11):2012–2021. doi: 10.1002/cncr.27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heron M. Deaths: Leading Causes for 2016. National Vital Statistics Reports. 2018 [PubMed] [Google Scholar]
- 3.Our World in Data: Cancer, (2 November 2019).
- 4.Misono S., Weiss N.S., Fann J.R., Redman M., Yueh B. Incidence of suicide in persons with cancer. J. Clin. Oncol. 2008;26(29):4731–4738. doi: 10.1200/JCO.2007.13.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robson A., Scrutton F., Wilkinson L., MacLeod F. The risk of suicide in cancer patients: a review of the literature. Psychooncology. 2010;19(12):1250–1258. doi: 10.1002/pon.1717. [DOI] [PubMed] [Google Scholar]
- 6.Bergen H., Hawton K., Kapur N., Cooper J., Steeg S., Ness J., Waters K. Shared characteristics of suicides and other unnatural deaths following non-fatal self-harm? A multicentre study of risk factors. Psychol. Med. 2012;42(4):727–741. doi: 10.1017/S0033291711001747. [DOI] [PubMed] [Google Scholar]
- 7.Fauske L., Bruland O.S., Grov E.K., Bondevik H. Cured of primary bone cancer, but at what cost: a qualitative study of functional impairment and lost opportunities. Sarcoma. 2015;2015 doi: 10.1155/2015/484196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fauske L., Lorem G., Grov E.K., Bondevik H. Changes in the body image of bone sarcoma survivors following surgical treatment–a qualitative study. J. Surg. Oncol. 2016;113(2):229–234. doi: 10.1002/jso.24138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paredes T., Canavarro M.C., Simões M.R. Anxiety and depression in sarcoma patients: emotional adjustment and its determinants in the different phases of disease. Eur. J. Oncol. Nurs. 2011;15(1):73–79. doi: 10.1016/j.ejon.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Rumsey N., Harcourt D. Body image and disfigurement: issues and interventions. Body Image. 2004;1(1):83–97. doi: 10.1016/S1740-1445(03)00005-6. [DOI] [PubMed] [Google Scholar]
- 11.Yang K., Zheng Y., Peng J., Chen J., Feng H., Yu K., Chen Y., Luo W., Yang P., Yang Y., Wu B. Incidence of death from unintentional injury among patients with cancer in the United States. JAMA Netw Open. 2020;3(2):e1921647. doi: 10.1001/jamanetworkopen.2019.21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balcı Şengül M.C., Kaya V., Şen C.A., Kaya K. Association between suicidal ideation and behavior, and depression, anxiety, and perceived social support in cancer patients. Med. Sci. Monit. 2014;20:329–336. doi: 10.12659/MSM.889989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang C.-K., Chang M.-C., Chen P.-J., Lin C.-C., Chen G.-S., Lin J., Hsieh R.-K., Chang Y.-F., Chen H.-W., Wu C.-L., Lin K.-C., Chiu Y.-J., Li Y.-C. A correlational study of suicidal ideation with psychological distress, depression, and demoralization in patients with cancer. Support. Care Cancer. 2014;22(12):3165–3174. doi: 10.1007/s00520-014-2290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cansever A., Uzun O., Yildiz C., Ates A., Atesalp A.S. Depression in men with traumatic lower part amputation: a comparison to men with surgical lower part amputation. Mil. Med. 2003;168(2):106–109. [PubMed] [Google Scholar]
- 15.Singh R., Hunter J., Philip A. The rapid resolution of depression and anxiety symptoms after lower limb amputation. Clin Rehabil. 2007;21(8):754–759. doi: 10.1177/0269215507077361. [DOI] [PubMed] [Google Scholar]
- 16.Camidge D.R., Stockton D.L., Frame S., Wood R., Bain M., Bateman D.N. Hospital admissions and deaths relating to deliberate self-harm and accidents within 5 years of a cancer diagnosis: a national study in Scotland, UK. Br. J. Cancer. 2007;96(5):752–757. doi: 10.1038/sj.bjc.6603617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surveillance Epidemiology and End Results (SEER) Program: Overview of the SEER Program (5 August 2020).
- 18.W.N. Koepsell TD, Epidemiologic Methods: Studying the Occurrence of Illness 2003 Oxford University Press New York, NY.
- 19.De Felice Francesca, Blanchard Pierre. Radiation-induced neurocognitive dysfunction in head and neck cancer patients. Tumori. 2017;103(4):319–324. doi: 10.5301/tj.5000678. [DOI] [PubMed] [Google Scholar]
- 20.Gan Hui K., Bernstein Lori J., Brown Jennifer, Ringash Jolie, Vakilha Mehrdad, Wang Lisa, Goldstein David, Kim John, Hope Andrew, O'Sullivan Brian, Waldron John, Abdul Razak Albiruni R., Chen Eric X., Siu Lillian L. Cognitive functioning after radiotherapy or chemoradiotherapy for head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011;81(1):126–134. doi: 10.1016/j.ijrobp.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Hameed Meera, Dorfman Howard. Primary malignant bone tumors–recent developments. Semin. Diagn. Pathol. 2011;28(1):86–101. doi: 10.1053/j.semdp.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Farfalli G.L., Albergo J.I., Ritacco L.E., Ayerza M.A., Muscolo D.L., Aponte-Tinao L.A. Oncologic and clinical outcomes in pelvic primary bone sarcomas treated with limb salvage surgery. Musculoskelet. Surg. 2015;99(3):237–242. doi: 10.1007/s12306-015-0379-7. [DOI] [PubMed] [Google Scholar]
- 23.R.J. Wilson, T.H. Freeman, Jr., J.L. Halpern, H.S. Schwartz, G.E. Holt, Surgical outcomes after limb-sparing resection and reconstruction for pelvic sarcoma: a systematic review, JBJS Rev. 6(4) (2018) e10. [DOI] [PubMed]
- 24.Siracuse B.L., Gorgy G., Ruskin J., Beebe K.S. What is the incidence of suicide in patients with bone and soft tissue cancer?: Suicide and sarcoma. Clin. Orthop. Relat. Res. 2017;475(5):1439–1445. doi: 10.1007/s11999-016-5171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kager Leo, Tamamyan Gevorg, Bielack Stefan. Novel insights and therapeutic interventions for pediatric osteosarcoma. Future Oncol. 2017;13(4):357–368. doi: 10.2217/fon-2016-0261. [DOI] [PubMed] [Google Scholar]
- 26.Bhatia R., Beckles V., Fox Z., Tirabosco R., Rezajooi K., Casey A.T. Osteosarcoma of the spine: dismal past, any hope for the future? Br. J. Neurosurg. 2014;28(4):495–502. doi: 10.3109/02688697.2013.869550. [DOI] [PubMed] [Google Scholar]
- 27.Leah P., Dower A., Vescovi C., Mulcahy M., Al Khawaja D. Clinical experience of intracranial chordoma – a systematic review and meta-analysis of the literature. J. Clin. Neurosci. 2018;53:6–12. doi: 10.1016/j.jocn.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 28.D'Amore T., Boyce B., Mesfin A. Chordoma of the mobile spine and sacrum: clinical management and prognosis. J. Spine Surg. 2018;4(3):546–552. doi: 10.21037/jss.2018.07.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song Paula H., Beyhaghi Hadi, Sommer Josh, Bennett Antonia V. Symptom burden and life challenges reported by adult chordoma patients and their caregivers. Qual. Life Res. 2017;26(8):2237–2244. doi: 10.1007/s11136-017-1544-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anwar M. Akhtar, El-Baba Chirine, Elnaggar Muhammed H., Elkholy Yasmeen O., Mottawea Mohamed, Johar Dina, Al Shehabi Tuqa S., Kobeissy Firas, Moussalem Charbel, Massaad Elie, Omeis Ibrahim, Darwiche Nadine, Eid A.H. Novel therapeutic strategies for spinal osteosarcomas. Semin. Cancer Biol. 2020;64:83–92. doi: 10.1016/j.semcancer.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Miyawaki Daisuke, Murakami Masao, Demizu Yusuke, Sasaki Ryohei, Niwa Yasue, Terashima Kazuki, Nishimura Hideki, Hishikawa Yoshio, Sugimura Kazuro. Brain injury after proton therapy or carbon ion therapy for head-and-neck cancer and skull base tumors. Int. J. Radiat. Oncol. Biol. Phys. 2009;75(2):378–384. doi: 10.1016/j.ijrobp.2008.12.092. [DOI] [PubMed] [Google Scholar]
- 32.Zhou H., Xian W., Zhang Y., Yang Y., Fang W., Liu J., Shen J., Zhang Z., Hong S., Huang Y., Zhang L. Suicide among cancer patients: adolescents and young adult (AYA) versus all-age patients. Ann. Transl. Med. 2019;7(22):658. doi: 10.21037/atm.2019.10.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bass S.B., Ruzek S.B., Gordon T.F., Fleisher L., McKeown-Conn N., Moore D. Relationship of Internet health information use with patient behavior and self-efficacy: experiences of newly diagnosed cancer patients who contact the National Cancer Institute's Cancer Information Service. J. Health Commun. 2006;11(2):219–236. doi: 10.1080/10810730500526794. [DOI] [PubMed] [Google Scholar]
- 34.Schiffman Joshua D., Csongradi Eric, Suzuki Lalita K. Internet use among adolescent and young adults (AYA) with cancer. Pediatr. Blood Cancer. 2008;51(3):410–415. doi: 10.1002/pbc.21616. [DOI] [PubMed] [Google Scholar]
- 35.Penn Anthony, Kuperberg Aura. Psychosocial support in adolescents and young adults with cancer. Cancer J. 2018;24(6):321–327. doi: 10.1097/PPO.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 36.Leung Yvonne W., Li Madeline, Devins Gerald, Zimmermann Camilla, Rydall Anne, Lo Chris, Rodin Gary. Routine screening for suicidal intention in patients with cancer. Psychooncology. 2013;22(11):2537–2545. doi: 10.1002/pon.3319. [DOI] [PubMed] [Google Scholar]
- 37.Coleman Karen J., Johnson Eric, Ahmedani Brian K., Beck Arne, Rossom Rebecca C., Shortreed Susan M., Simon Greg E. Predicting suicide attempts for racial and ethnic groups of patients during routine clinical care. Suicide Life Threat. Behav. 2019;49(3):724–734. doi: 10.1111/sltb.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreno-Betancur M., Sadaoui H., Piffaretti C., Rey G. Survival analysis with multiple causes of death: extending the competing risks model. Epidemiology. 2017;28(1):12–19. doi: 10.1097/EDE.0000000000000531. [DOI] [PubMed] [Google Scholar]
- 39.Ivey-Stephenson Asha Z., Crosby Alex E., Jack Shane P.D., Haileyesus Tadesse, Kresnow-Sedacca Marcie-jo. Suicide trends among and within urbanization levels by sex, race/ethnicity, age group, and mechanism of death – United States, 2001–2015. MMWR Surveill. Summ. 2017;66(18):1–16. doi: 10.15585/mmwr.ss6618a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu C.H., Stevens C., Wong S.H.M., Yasui M., Chen J.A. The prevalence and predictors of mental health diagnoses and suicide among U.S. college students: implications for addressing disparities in service use. Depress Anxiety. 2019;36(1):8-. doi: 10.1002/da.22830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data analyzed in this study were from the Surveillance, Epidemiology and End Results database, which was publicly available datasets.